Until relatively recently only one drug hydroxyurea was approved by the US Food and Drug Administration to ameliorate disease severity. Adakveo and Oxbryta could be revolutionary treatments but each costs about 100000 per year and must be taken for life.
 Fda Approves Oxbryta Voxelotor The First Medicine Specifically Targeting The Root Cause Of Sickle Cell Disease Tif
Fda Approves Oxbryta Voxelotor The First Medicine Specifically Targeting The Root Cause Of Sickle Cell Disease Tif
Glutamine sickle cell 5 gram oral powder packet.

Sickle cell drugs. A blood and bone marrow transplant is currently the only cure for sickle cell disease but there are effective treatments that can reduce symptoms and prolong life. Food and Drug Administration granted accelerated approval to Oxbryta voxelotor for the treatment of sickle cell disease SCD in adults. But in the past sickle cell drugs had to reduce the number of painful crises in order to win approval and voxelotors skeptics say its data are simply not compelling.
There are many types of street drugs. Despite Food and Drug Administration FDA approval of hydroxyurea to reduce the frequency of vaso-occlusive episodes sickle cell disease SCD has continued to be treated primarily with analgesics for pain relief. Antibodies Monoclonal Humanized administration dosage.
FDA approves crizanlizumab-tmca for sickle cell disease On November 15 2019 Food and Drug Administration approved crizanlizumab-tmca ADAKVEO Novartis to reduce the frequency of. Anemia Sickle Cell drug therapy. Multiple other drugs L-glutamine crizanlizumab.
The severity of the complications that occur with this disorder are widely variable but overall mortality is increased and life expectancy decreased when compared to the general population. Sickle cell disease SCD is a potentially devastating condition that is caused by an autosomal recessive inherited hemoglobinopathy which results in the vaso-occlusive phenomena and hemolysis. Many of them have side effects that can worsen the symptoms of the disease.
List of drugs used to treat the medical condition called Sickle Cell Anemia. This leads to a rigid sickle-like shape under certain circumstances. Oxbryta 500 mg tablet Sickle Hemoglobin HbS Polymerization Inhibitor.
Zinc may decrease how often you have pain. 64 Zeilen Drugs used to treat Anemia Sickle Cell. The next GBT drug in the pipeline is inclacumab an antibody designed to target P-selectin a target in sickle cell disease that is known to reduce the incidence of vaso-occlusive crisis a painful.
Antibodies Monoclonal Humanized adverse effects. It also appears promising for. Theres a famous saying in.
Even prescription drugs can be harmful if they are not taken as prescribed by the doctor. Click on the drug to find more information including the brand namesdoseside-effects adverse events when to take. November 25 2019 Today the US.
Select drug class All drug classes antimetabolites 4 miscellaneous uncategorized agents 4 vitamins 10 nutraceutical products 3 Rx. Problems in sickle cell disease typically begin around 5 to 6. In June the FDA approved the drug Endari L-glutamine oral powder the first new drug for sickle cell disease in almost 20 years.
Folic acid may help prevent blood vessel problems that can come with sickle cell anemia. All street drugs those not prescribed by a doctor can cause health problems in a person with sickle cell disease. Zinc may decrease how often you have pain.
The drugs used in treatment of sickle cell disease SCD include antimetabolites analgesics antibiotics vaccines and nutritional agents. Sickle cell disease SCD is a group of blood disorders typically inherited from a persons parents. This brochure will discuss marijuana.
It results in an abnormality in the oxygen-carrying protein haemoglobin found in red blood cells. Your healthcare team will work with you on a treatment plan to. Two New Drugs Help Relieve Sickle-Cell Disease.
But Who Will Pay. The most common type is known as sickle cell anaemia SCA. The following list of medications are in some way related to or used in the treatment of this condition.
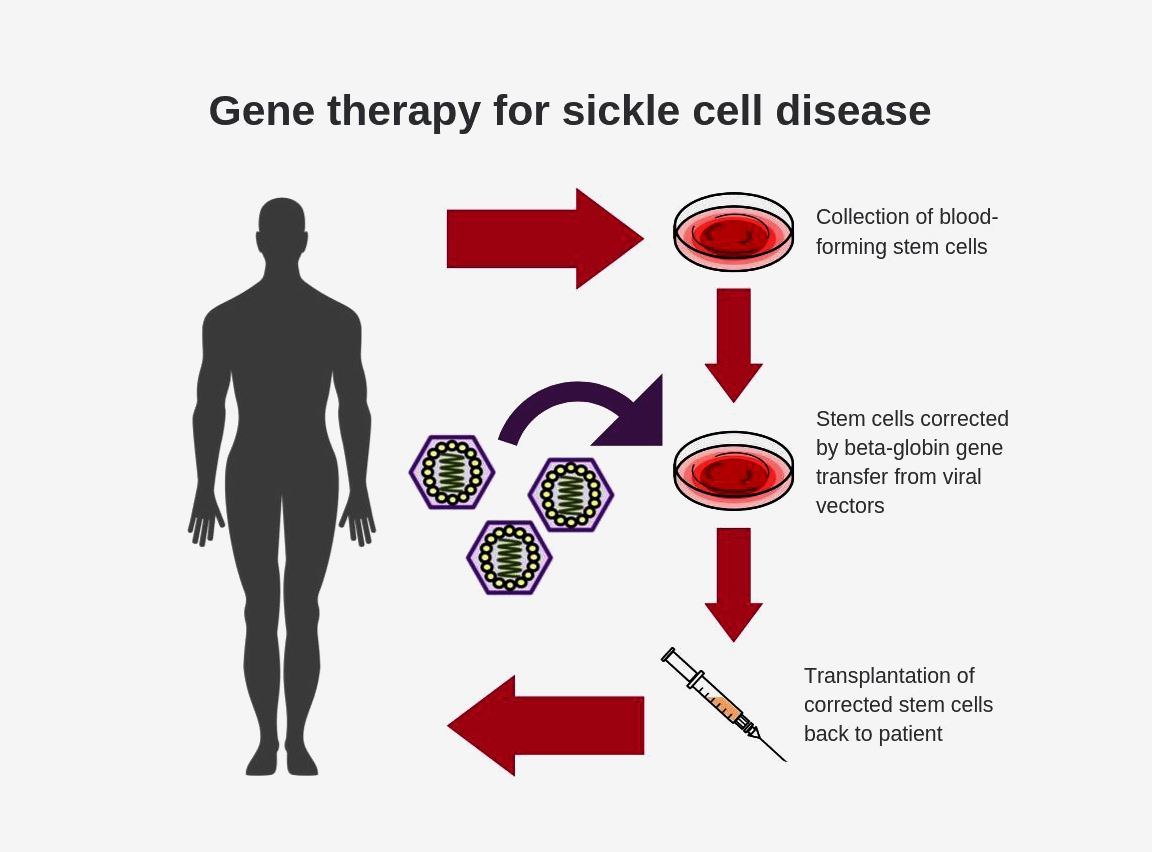
Voxelotor 500 mg tablet. Sickle cell disease is a lifelong illness. SCD can be cured using hematopoietic stem cell transplantation and possibly gene therapy but these treatment approaches are not available to most patients the majority of whom reside in low- and middle-income countries.