Generally the importation or reimportation of a prescription drug that does not meet Food and Drug Administration FDA requirements is prohibited. In practice this means a person with stage 4 colon cancer might be allowed to obtain a chemotherapy candidate being tested in Europe but not America.
 The Reality Of Prescription Drug Importation Rxbenefits
The Reality Of Prescription Drug Importation Rxbenefits
The policy debate largely has been.

Importation of prescription drugs. September 28 2020 - The FDA recently finalized a rule that would allow prescription drug importation from Canada in FDA-authorized programs. Requiring an Importer to file a separate Pre-Import Request for each shipment would not. Prescription drugs including biological products imported under the pathway described in the final guidance could be available to patients in a variety of settings including hospitals health.
Could also affect foreign markets and pharmaceutical manufacturers might alter their pricing strategies. An Importer could also choose to send one eligible prescription drug covered by a Pre-Import Request in multiple shipments. Reimportation of prescription drugs is back as an issue but only because Democrats seek to distract from the effort to repeal and replace Obamcare according to PoliticoBy importation we refer to the ability of American consumers to buy their prescription drugs from overseas rather than from domestic sources and particularly to large-scale importation such as US drug.
It is not clear at all that importation or re-importation of prescription drugs should be banned. First even if you believe in the nanny-state justification the government could be content with providing information to consumers as to which drugs it deems safe. An Importer could choose to send each eligible prescription drug covered by a Pre-Import Request in a separate shipment for example.
Even then the medication cleared for importation must. On December 23 2019 FDA issued a proposed rule to allow importation of eligible prescription drugs from Canada 84 Federal Register 70796. The Prescription Drug Marketing Act also prohibits non-manufacturers from importing prescription drugs so even drugs made in the United States approved by the FDA and exported cannot be re-imported by wholesalers or other manufacturers.
The policy debate has centered on. Generally the importation or reimportation of a prescription drug that does not meet Food and Drug Administration FDA requirements is prohibited. Prescription Drug Importation In the context of rising drug prices the possibility of importing prescription drugs from other countries at lower prices is again being debated.
1 Importer The term importer means a pharmacist or wholesaler. Prescription Drug Importation In the context of rising drug prices the possibility of importing prescription drugs from other countries at lower prices is again being debated. Regulatory sovereignty to foreign governments threatens our intellectual property protections and slows the pharmaceutical innovation that is driving Americas economy and.
Product identifier on most prescription drug packages and outlines steps to build an electronic interoperable system for identifying and tracing prescription drugs as they are distributed in the United States14 Importing drugs into the US. As stated on the presidents healthcare plan Biden will allow consumers to import prescription drugs from other countries as long as the US. Has become a popular idea among consumers some state and federal legislators and even the Trump Administration.
FDAs no commercialization or promotion rule pretty much limits prescription drug importation by individuals to experimental drugs for imminently fatal conditions. With the increasingly high cost of prescription drugs importing drugs into the US. 2 Pharmacist The term pharmacist means a person licensed by a State to practice pharmacy including the dispensing.
The final rule part of the White Houses Safe Importation Action Plan fulfills the aspect of the previous executive order which will allow states to import certain prescription drugs from Canada. 3 Prescription drug The term prescription drug means a drug subject to section 353 b of this. Importation yields US.
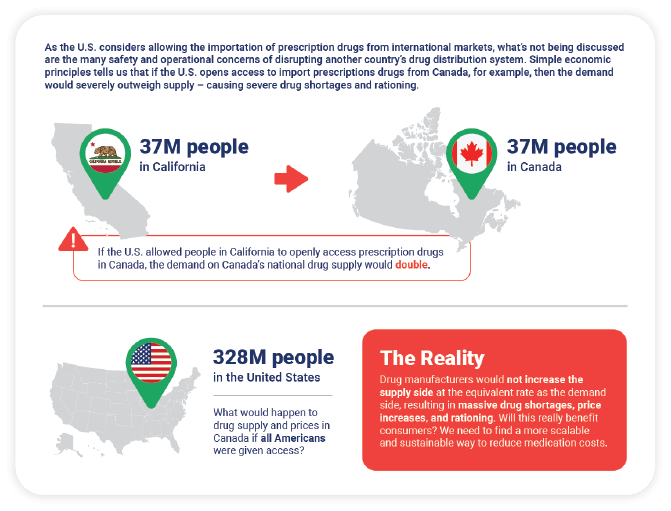
Department of Health and Human Services has certified that those drugs are safe. However there are multiple risks and concerns of this solution that are not being widely discussed. A facilitating grants to individuals of waivers of the prohibition of importation of prescription drugs provided such importation poses no additional risk to public safety and results in lower costs to American patients pursuant to section 804 j 2 of the Federal Food Drug and Cosmetic Act FDCA 21 USC.