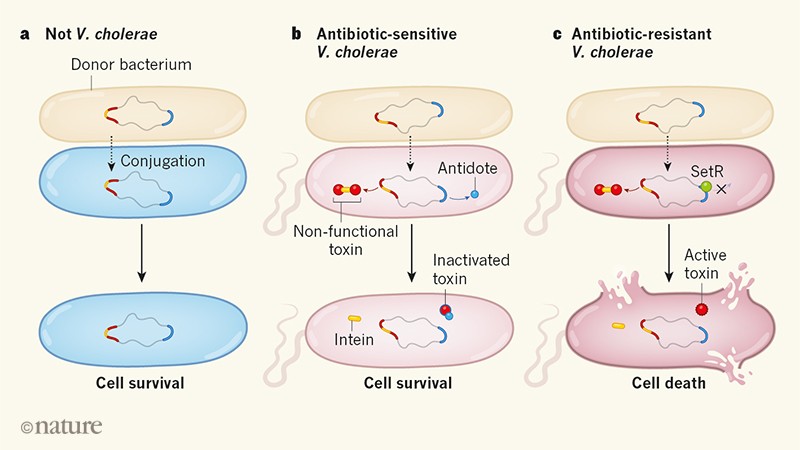
Publications Features plus. Among women aged 30-64 years the unadjusted drug overdose death rate increased 260 from 67 deaths per 100000 population 4314 total drug overdose deaths in 1999 to 243 18110 in 2017.
 Drug Overdose Deaths Declined In 2018 Mdedge Psychiatry
Drug Overdose Deaths Declined In 2018 Mdedge Psychiatry
In recent years increases in opioid-involved overdose deaths have been driven primarily by deaths involving synthetic opioids other than.
Drug overdose deaths by state 2017. In just the past year Wisconsin had 12 percent more unintentional drug deaths for. 1 The decline follows an increasing trend in the rate from 61 in 1999 to 217 in 2017. Centers for Disease Control and Prevention National Center for.
We examined if a shift has taken place where rates of Black fatal overdoses have now surpassed Whites in the state. In the US there were 67367 drug overdose deaths reported in 2018 41 fewer deaths than in 2017. What States Need to Know about PDMPs.
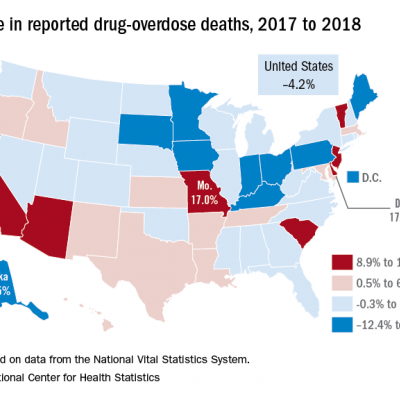
The following list ranks the 50 states and the District of Columbia by how sharply overdose deaths from all drugs fell from 2017 to 2018The list uses data from the Center for Disease Control and. Results presented here derive from vital. 513 deaths per 100000.
In 2017 the rate of drug overdose deaths among males was twice as high as the rate among females age-adjusted rates. 2017 Drug Overdose Death Rates. Back to Drug Overdose Deaths Webpage.
Opioids were involved in 46802 a rate of 146 overdose deaths in 2018nearly 70 of all. Prescription opioid-related deaths increased between 1999 and 2017. Results-Among drug overdose deaths in 2017 that mentioned at least 1 specific drug on the death certificate the 10 drugs most frequently involved included fentanyl heroin cocaine.
371 deaths per 100000 population in 2017. Of drug overdose deaths from 142 deaths age- adjusted rate. Among 70237 drug overdose deaths in 2017 47600 678 involved an opioid.
Among 70237 drug overdose deaths in 2017 47600 678 involved an opioid. Pleaded guilty in March to one count of possession with intent to distribute fentanyl in November 2017. For females there were 149 deaths in 2008 and 363 in 2017 a 144 percent increase.
1 The decline follows an increasing trend in the rate from 61 in 1999 to 217 in 2017. Of the 70237 drug overdose deaths in the United States in 2017 approximately two thirds 47600 involved an opioid 1. Drug Overdose Mortality by State.
More than 72000 Americans died of drug overdoses in 2017 up nearly 7 from 2016 according to preliminary data. Drug overdose fatality rates were calculated by number of deaths per year per 100000 population from 2012 to 2019 in Connecticut. When the number of deaths is small.
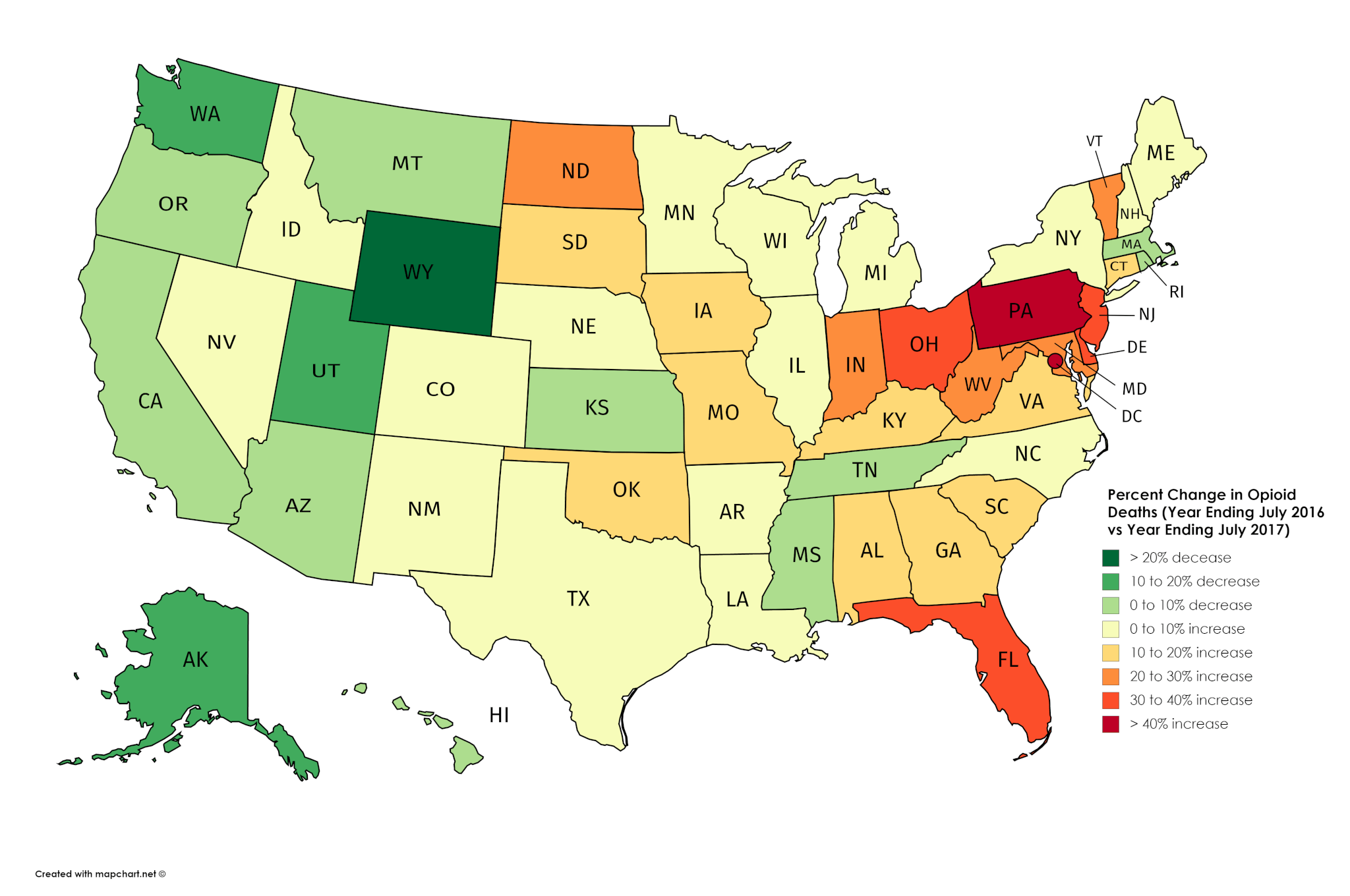
In the earlier drug case related to an overdose death defendant Derrick L. 1 In 2019 70630 drug overdose deaths occurred in the United States. From 2013 to 2017 drug overdose death rates increased in 35 of 50 states and DC and significant increases in death rates involving synthetic opioids occurred in 15 of 20 states likely driven by IMF 23.
Drug-Involved Overdose Deaths. The age-adjusted rate of overdose deaths increased by over 4 from 2018 207 per 100000 to 2019 216 per 100000. That represents a twofold increase over a decade the CDC says.
There were 281 unintentional drug overdose deaths for males in 2008 and 669 in 2017 a 138 percent increase. The age-adjusted rate declined by 46 to 207 per 100000 standard population. The age-adjusted rate declined by 46 to 207 per 100000 standard population.
Deaths involving more than one drug were counted in all relevant drug categories ie the same death could be counted in more than one drug category. Opioids were involved in 46802 a rate of 146 overdose deaths in 2018nearly 70 of all. Back to Drug Overdose Deaths Webpage.
Drug-Involved Overdose Deaths. States are categorized from highest rate to lowest rate. The number and rate of deaths involving antidepressants benzodiazepines cocaine heroin and synthetic opioids each increased during this period.
153 deaths per 100000 population in 2012 to 346 deaths age-adjusted rate. Although adjusted for differences in age-distribution and population size rankings by state do not take into account other state specific population characteristics that may affect the level of mortality. 2017 Tennessee Drug Overdose Deaths Page 1 Introduction and purpose The purpose of this brief report is to describe drug overdose deaths in Tennessee in 2017 with an emphasis on providing useful data to stakeholders of Tennessee Department of Health TDH at the regional and county levels.
From 2016 to 2017 overdose deaths involving all opioids and synthetic opioids increased but deaths. In addition changes in death rates from 2016 to 2017 involving all opioids and opioid subcategories were examined by demographics county urbanization levels and by 34 states and DC. In the US there were 67367 drug overdose deaths reported in 2018 41 fewer deaths than in 2017.
Nearly 841000 people have died since 1999 from a drug overdose. Historically Blacks and Hispanics have had lower opioid-involved overdose death rates in Connecticut CT.