Eli Lilly and Co GSK JJ Pfizer Teva Pharma. Aimovig is the first and only FDA-approved treatment that blocks the CGRP receptor to help prevent migraine.
 How Can Teva S Migraine Drug Face Off Amgen And Lilly Put Neurologists First Dtc Last Executive Says Fiercepharma
How Can Teva S Migraine Drug Face Off Amgen And Lilly Put Neurologists First Dtc Last Executive Says Fiercepharma
Amgen Inc AstraZeneca Plc Bausch Health Companies Inc.

Amgen migraine drug. The new drug Aimovig was approved by the FDA on Thursday. Amgen and Novartis on Thursday secured Food and Drug Administration approval for Aimovig the first regulatory thumbs up for a new class of migraine treatments called calcetonin gene-related peptide CGRP inhibitors. Neulasta pegfilgrastim is used to treat neutropenia.
Will charge a lower-than-expected 6900 a year for a novel migraine treatment that investors hope will become the biotechnology giants next blockbuster. Aimovig a once-a-month CRGP receptor-blocking antibody from Amgen NASDAQAMGN and Novartis led this class with its approval to prevent migraines from the US. Aimovig is the first drug specifically designed to prevent migraine a condition that until now has been treated with decades-old therapies primarily intended to treat other afflictions Anthony.
Aimovig is a prescription medicine used to help prevent migraine in adults. Another one of Amgens top-selling drugs is called Neulasta. The drug is available for self-administering.
Food and Drug Administration approval on Thursday for the first drug to prevent migraine headaches in adults. Amgen won US. The drug Aimovig which is given monthly by self-injection will have a.
Amgen takes the issue of counterfeit drugs very seriously and is committed to the highest standards of drug quality and patient safety. Market to Reach 69 Trillion by 2027 - Key Players are Allergan Amgen Inc. 23 Aimovig co-commercialized in the US.
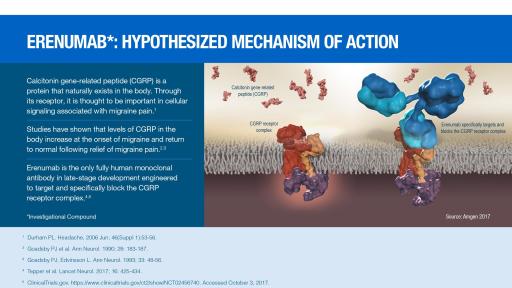
It acts as a white blood cell stimulant. The AMG-334 drug is erenumab which is designed to block the calcitonin gene-related peptide CGRP receptor that is believed to be intricately tied to migraines. Food and Drug Administration in.
Global Migraine Drugs Market Report 2021. Food and Drug Administration FDA approved Aimovig erenumab-aooe for the prevention of migraine headaches. Safety Data Sheets Safety data sheets provide information for healthcare professionals and others seeking information on implications of the exposure to.
In May 2018 the US. Aimovig heads to market with a broad label cleared for the preventive treatment of migraine in adults. The drug was commercialized in a partnership between Novartis and Amgen.
20 hours agoMigraine drugs refer to medications that are used to reduce the severity of migraines and prevent future attacks. Aimovig is a newly approved agent specifically designed to prevent migraine by blocking the receptor of the calcitonin gene-related peptide CGRP which plays a. Migraine is a highly debilitating disease that has a profound and limiting impact on peoples lives including time spent with family and friends or at work.
The drug works by blocking a compound called calcitonin gene-related peptide receptor CGRP-R which is believed to play a critical role in migraine. Reuters - Amgen Incs push to get patients on its new 575 a month migraine drug before competition emerges in September is facing barriers from insurers. Amgens product pipeline will change over time as molecules move through the drug development process including progressing through clinical phases to licensure and market returning to strategic partners being outlicensed or failing in clinical trials to demonstrate efficacy safety or to deliver a commercially viable product due to the.
A 2015 deal gave Novartis co-marketing rights in the US. By Amgen and Novartis is the first and only FDA-approved treatment that prevents migraine by targeting the calcitonin gene-related peptide CGRP receptor. In addition to commercial rights everywhere else except Japan.
A migraine is a severe neurological disease with a debilitating headache characterized by an intense throbbing or pulsing in one area of the head. INDIANAPOLIS Reuters - Eli Lilly and Co has pulled ahead in a three-way race with Amgen Inc and Teva Pharmaceutical Industries Ltd in attracting new patients to a new class of migraine. Amgen Incs headquarters building is.
Its the first medicine in a new class thats designed to reduce the number of migraines. More than 300000 people have been prescribed Aimovig to help prevent migraine.

 Novartis Amgen Tout Aimovig S Benefits With Real World And Long Term Data Fiercepharma
Novartis Amgen Tout Aimovig S Benefits With Real World And Long Term Data Fiercepharma
Express Scripts Covers Amgen And Lilly But Snubs Teva S New Migraine Drug Biospace
 Amgen Novartis Set To Launch Migraine Drug Aimovig Next Week After Fda Approval
Amgen Novartis Set To Launch Migraine Drug Aimovig Next Week After Fda Approval
 Fda Approves Amgen S Migraine Drug
Fda Approves Amgen S Migraine Drug
 Amgen Novartis Clinch Fda Nod On Migraine Drug Aimovig Thestreet
Amgen Novartis Clinch Fda Nod On Migraine Drug Aimovig Thestreet
 Amgen S Aimovig Won Its First In Class Migraine Nod Will Payers Step Up To The 6 900 Price Fiercepharma
Amgen S Aimovig Won Its First In Class Migraine Nod Will Payers Step Up To The 6 900 Price Fiercepharma
 Xconomy Teva Wins Fda Nod For Migraine Drug Sets Price In Line With Amgen S
Xconomy Teva Wins Fda Nod For Migraine Drug Sets Price In Line With Amgen S

 Amgen S New Migraine Drug Will Cost 30 Percent Less Than Wall Street Expected Scientific American
Amgen S New Migraine Drug Will Cost 30 Percent Less Than Wall Street Expected Scientific American
 Aimovig Erenumab Phase 3 Strive Data Published In The New England Journal
Aimovig Erenumab Phase 3 Strive Data Published In The New England Journal
Mind Blowing Amgen S Aimovig Halves Length Of Migraine Attacks In Phase Iii Study Biospace
 Eli Lilly And Company Lilly S New Migraine Drug Pulls Ahead Of Amgen In Fierce Battle For New Prescriptions Health News Et Healthworld
Eli Lilly And Company Lilly S New Migraine Drug Pulls Ahead Of Amgen In Fierce Battle For New Prescriptions Health News Et Healthworld
No comments:
Post a Comment
Note: Only a member of this blog may post a comment.