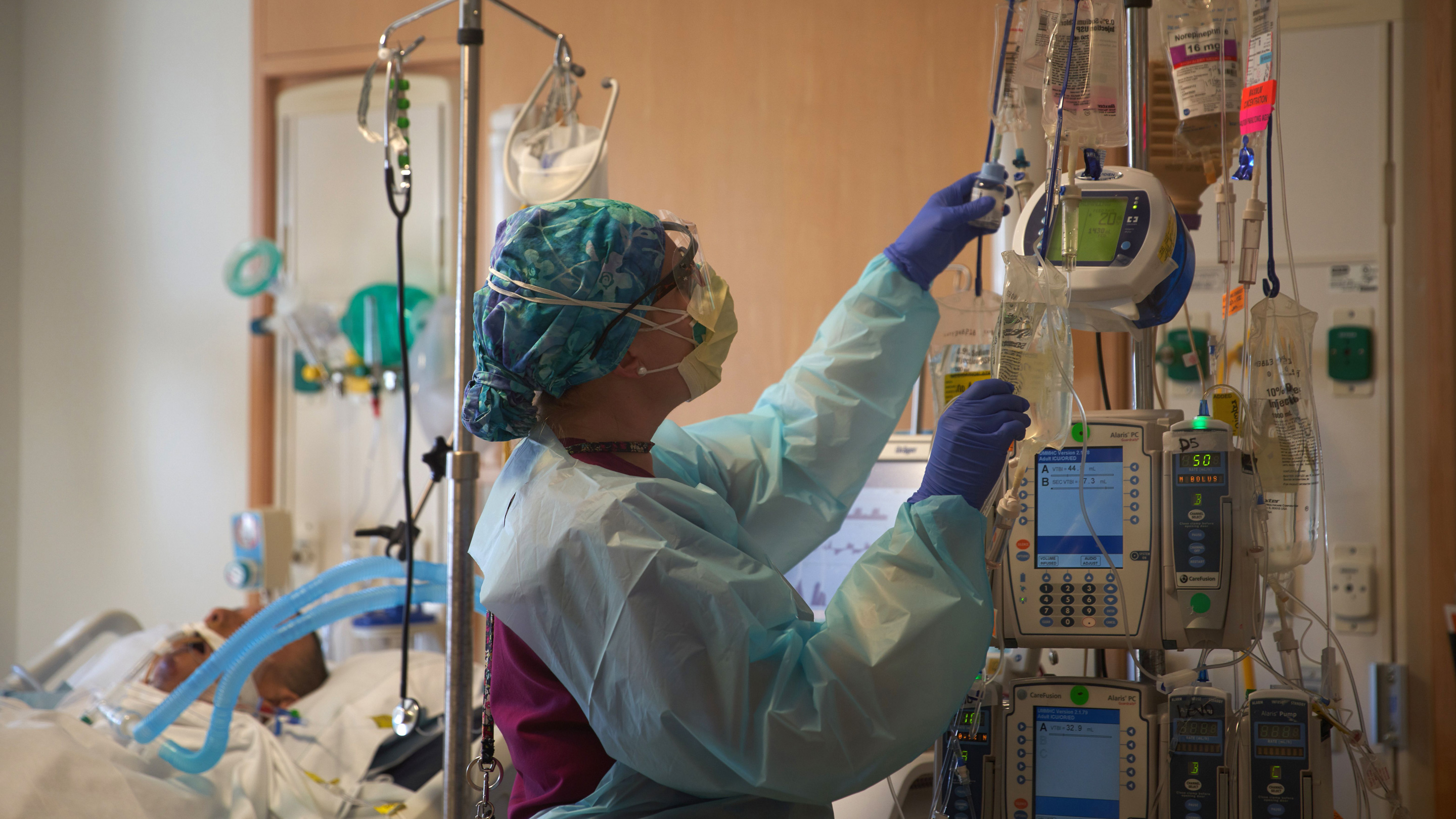
Oxbryta a once-daily pill from Global Blood Therapeutics Inc blocks a process in blood cells that can lead to anemia and organ damage hallmarks of sickle cell disease. The FDA on Friday approved a new drug that reduces complications associated with sickle cell disease the first approved for the disorder in more than 20.
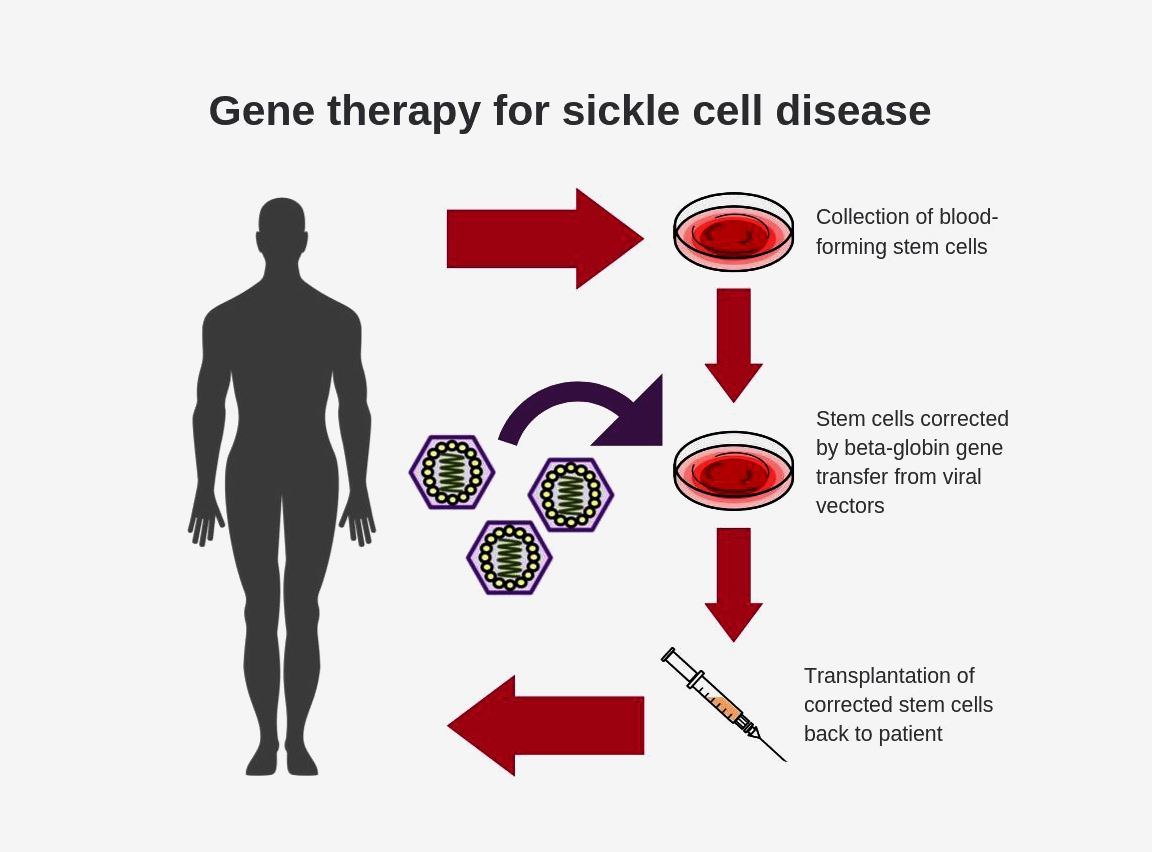 Nih Researchers Create New Viral Vector For Improved Gene Therapy In Sickle Cell Disease National Institutes Of Health Nih
Nih Researchers Create New Viral Vector For Improved Gene Therapy In Sickle Cell Disease National Institutes Of Health Nih
FDA Approves Crizanlizumab A New Drug For Sickle Cell Disease.
New drugs for sickle cell anemia. Benzaldehydes administration. The Food and Drug Administration approved Novartis AGs Adakveo for patients 16 and older. Adakveo and Oxbryta could be revolutionary treatments but each costs about 100000 per year and must be taken for life.
November 25 2019 Today the US. Osunkwo says many patients were thrilled to have a nonchemotherapeutic option available. Food and Drug Administration granted accelerated approval to Oxbryta voxelotor for the treatment of sickle cell disease SCD in adults.
FDA on Monday approved the sickle cell disease drug voxelotor OxbrytaGlobal Blood Therapeutics adding to a new wave of treatments that promise relief from the life-threatening blood disorder that largely afflicts African-Americans. Regulators on Friday approved a new medicine that can help reduce extremely painful sickle cell disease flare-ups. Adakveo is approved to help reduce frequency of VOCs among members aged 16 years and older with sickle cell disease and prior vasooclusive crises.
However the medical community received the drug with some skepticism. The amino acid L-glutamine Endari became the second drug approved for sickle cell in 2017. Antibodies Monoclonal Humanized adverse effects.
64 рядків Drugs used to treat Anemia Sickle Cell. The drug blocks a process in blood cells that can lead to anemia and organ damage hallmarks of sickle cell disease. I am a writer journalist professor systems modeler computational and.
The patients were randomly assigned in a 21 ratio to receive either l-glutamine or placebo. Regulators on Friday approved a new medicine that can help reduce extremely painful sickle cell disease flare-ups. Indicated for patients 5 years of age and older Dr.
But Who Will Pay. Opinions expressed by Forbes Contributors are their own. Oxbryta treatment is approved for members 12 years of age and older with sickle cell disease and a pretreatment hemoglobin level of 105 gdL or less.
It contains an antibody that blocks a protein in the blood vessels that binds to sickle cells causing pain and inflammation when the sickle cells block blood flow. The Food and Drug. KA A double-blind placebo-controlled phase 3 study evaluated 230 patients with sickle cell anemia or sickle ß 0-thalassemia who were at least 5 years of age and had experienced at least 2 acute pain episodes in the previous 12 months NCT01179217.
Yutaka Niihara CEO of Emmaus Medical has spent much of his career developing Endari a drug now approved by the FDA to treat sickle cell anemia. Antibodies Monoclonal Humanized administration dosage. Hydroxyurea therapy for sickle cell anemia.
Food and Drug Administration today approved Endari L-glutamine oral powder for patients age five years and older with sickle cell disease to reduce severe complications associated with. The following list of medications are in. The first new drug in decades to be approved for treating sickle cell disease is L-glutamine marketed as Endari by Emmaus Life Sciences.
FDA OKs First New Treatment for Sickle Cell in Almost 20 Years. This amino acid is. Two New Drugs Help Relieve Sickle-Cell Disease.
Anemia Sickle Cell drug therapy. Adakveo crizanluzumab developed by Novartis is also approved as a treatment for sickle cell disease. Its the second drug to treat sickle cell anemia to come to the market in the past month.
In mid-November the Novartis drug Adakveo was approved by the FDA to reduce the frequency of sickle.