What is the Cologuard test. Scott screened for his family.
 Cologuard Competitor Is No Big Threat Exact Sciences Ceo Says Business News Madison Com
Cologuard Competitor Is No Big Threat Exact Sciences Ceo Says Business News Madison Com
Defending champion Bernhard Langer also shot a 69 and is tied for 11th at 6 under.

Who makes cologuard. Building on the success of Cologuard and Oncotype tests Exact Sciences is investing in its product pipeline to support patients throughout their cancer diagnosis and treatment. The Colorguard test is different from a colonoscopy because you can do it at home and you do not need to prepare by fasting or discontinuing medications before or after the test. The Food and Drug Administration on Monday approved the first screening test for colon cancer that uses patients DNA to help spot potentially deadly tumors and growths.
Cologuard was designed to help more people get screened and its doing just that. WebMD asked these two experts -- who have no ties to the company making Cologuard -- and the test maker for more details. Exact Sciences the maker of the Cologuard DNA-based test to screen for colon cancer headquartered in Madison Wisconsin.
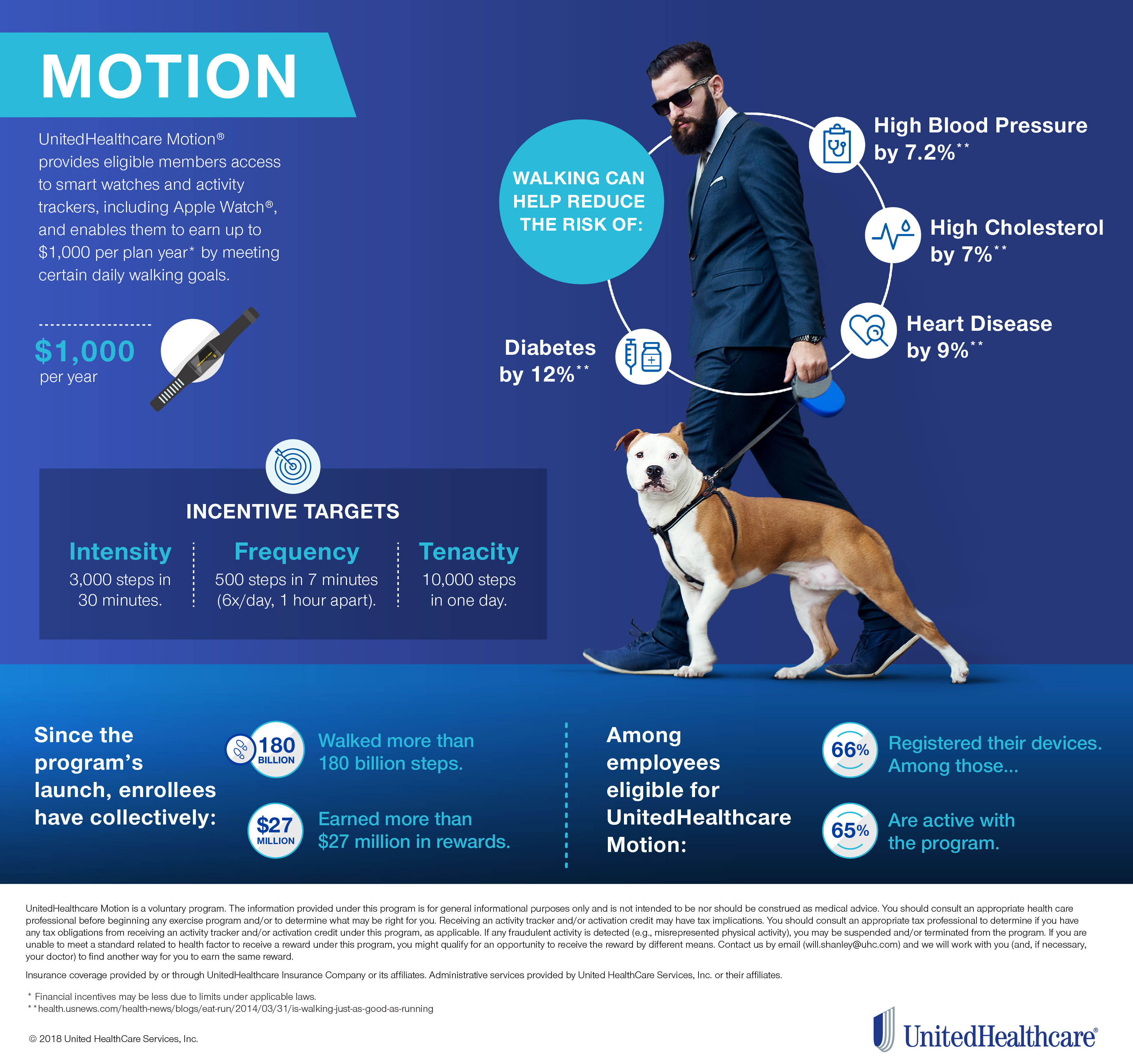
And with the recent announcement of a marketing partnership with Pfizer that will increase the size of the sales force promoting Cologuard to primary care physicians and hospitals this may be. Cologuard is the recently Food and Drug Administration FDA-approved stool deoxyribonucleic acid DNA screening test for detecting colon cancer. Cologuard is intended to screen adults 45 and older at average risk for colorectal cancer.
George Budwell has been writing about healthcare and biotechnology companies at the Motley Fool since 2013. Do not use if you have had adenomas have inflammatory bowel disease and certain hereditary syndromes or a personal or family history of colorectal cancer. Under terms of the nationwide agreement Pfizer will continue to provide marketing and related support for Cologuard and join Exact Sciences efforts to.
Shares of the company best known for its Cologuard colorectal cancer screening test have skyrocketed nearly 6000. Ordering a colonoscopy and waiting for the results takes time. His primary interests are novel small molecule drugs next generation vaccines and cell.
Food Drug Administration FDA-approved non-invasive stool DNA test used to screen for colorectal cancer in average. It costs 649 and is. Furyk whos making his Cologuard Classic debut is tied for 15th at 5 under.
Cologuard has definite benefits some downsides Mary Fischer is one of three nurses who had a colonoscopy in 2005 in an effort to bring attention to the importance of the procedure. Its recommended every three years. The Cologuard test from.
Cologuard is the only stool-DNA screening test for detecting colon cancer that is approved by the Food and Drug Administration FDA. Using a single stool sample collected at home Cologuard has been shown to be a highly sensitive noninvasive test which finds both cancer and precancer including 69 percent of polyps with high grade. It Its recommended every three years.
Cologuard looks for changes in your. No matter how you look at it Exact Sciences NASDAQEXAS has been a big winner. Approximately half of all Cologuard users age 50 to 75 have never been screened before.
Cologuard has emerged as a major weapon in the battle against colorectal. Primary care doctors can meet that quality metric by ordering a Cologuard making. The newest stool test is called Cologuard.
Cologuard is a US.