For results by phone call the BCCDC COVID-19 Negative Test Result line at 1-833-707-2792. The US Centers for Disease Control and Prevention acknowledged on Friday it had mixed together results from viral and antibody coronavirus tests on its website.
 Testing And International Air Travel Cdc
Testing And International Air Travel Cdc
30 false positive and 20 false negative confirms Conn.

Cdc test results. From another country to use home tests for their required negative test results. Talk to a healthcare provider about getting tested. CDCs home for COVID-19 data.
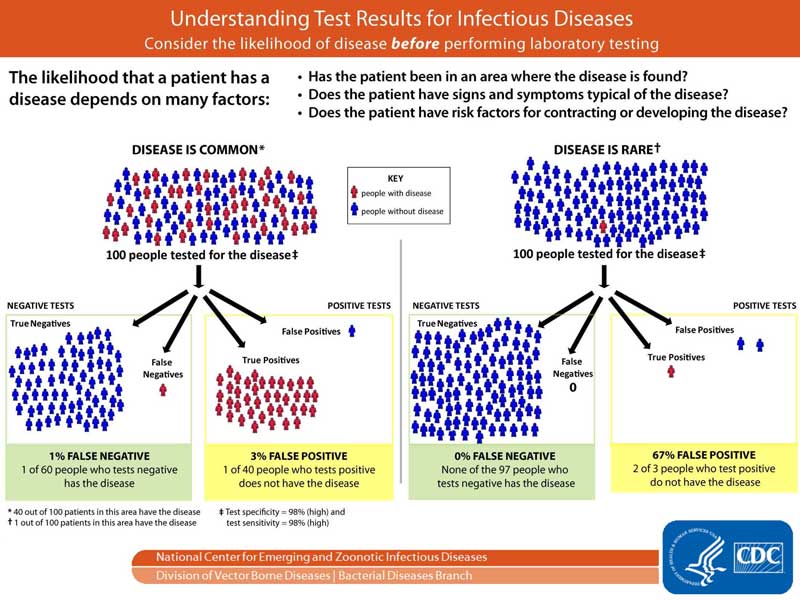
Each test requires a minimum level of viral load in order to produce a positive result. A 90 sensitive test will correctly identify 90 of infections missing the other 10 a false negative. Centers for Disease Control and Prevention CDC on Thursday publicly released guidelines for airlines to verify negative COVID-19 test results and Travelers who present a negative COVID-19 test or proof of recovery to board a flight back to the US.
What Your Test Results Mean pdf icon If you test positive stay home. The public-radio station WLRN in Miami first reported that the CDC was mixing viral and antibody test results. Negative test results in persons with known SARS-CoV-2 exposure suggest no current evidence of infection.
The CDC updated its testing requirements Friday to allow travelers flying into the US. The line is open daily from 830am-430pm. But this medical test is often inaccurate.
Positive test results allow for identification and isolation of infected persons as well as a case interview to identify and notify the cases close contact s of exposure and the need to quarantine. But in the ensuing weeks antibody tests expanded and CDC officials realized they had a growing number of those mixing in with the viral results the CDCs Dr. CDC recommends that anyone with any signs or symptoms of COVID-19 get tested regardless of vaccination status or prior infection.
Pathologist in peer-reviewed report. Sometimes the test can result in a false positive wrongly suggesting a patient does have coronavirus-fighting antibodies or. Two types of viral tests are used.
Scientists say it could create a. Viral tests can be performed in a laboratory at a testing site or at home or anywhere else. Widely used CDC Coronavirus test results.
Results are generally available after 48 hours but this. Are subject to meeting these requirements. HIV is spread through unprotected sex and drug-injecting behaviors so people who engage in these behaviors should get tested more often.
Parents can call to receive results for their children. A viral test checks specimens from your nose or your mouth to find out if you are currently infected with the virus that causes COVID-19. Visualizations graphs and data in one easy-to-use website.
Persons suspected of COVID-19 illness who test positive by direct viral detection methods for SARS-CoV-2 eg polymerase chain reaction or antigen detection tests typically begin to develop measurable antibody 7-14 days after illness onset and by 3 weeks most persons will test positive for antibody. A telehealth provider affiliated with the test manufacturer must be present remotely in real time to supervise the travelers self-test. Even relatively high sensitivity rates can produce high rates of false negatives in populations with low incidence rates.
Nucleic acid amplification tests NAATs and antigen tests. CDC recommends that health care providers test everyone for HIV between the ages of 13 and 64 at least once as part of routine health care. Who should get tested.
A viral test may tell you if you have a current infection with the virus that causes COVID-19. The CDC combines results of a test that spots people who are actively infected with results from another one that looks for antibodies. The CDC 2019-Novel Coronavirus 2019-nCoV Real-Time RT-PCR Diagnostic Panel is a real-time RT-PCR test intended for the qualitative detection of.
But the home tests have to be either a nucleic acid amplification test NAAT or an antigen test approved for emergency use by the Food and Drug Administration. If you get tested because you have symptoms or were potentially exposed to the virus you should stay away from others pending test results and follow the advice of your health care provider or a public health professional.
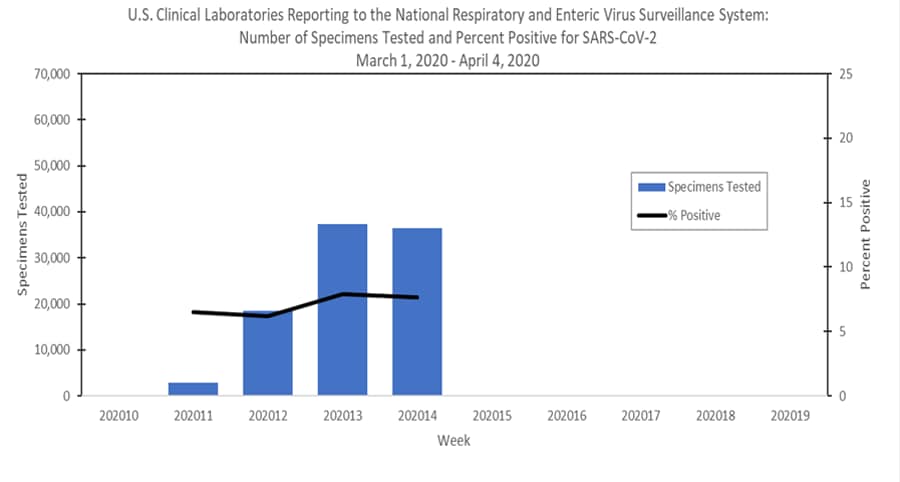 U S Clinical Laboratories Reporting Sars Cov 2 Test Results To Cdc Cdc
U S Clinical Laboratories Reporting Sars Cov 2 Test Results To Cdc Cdc
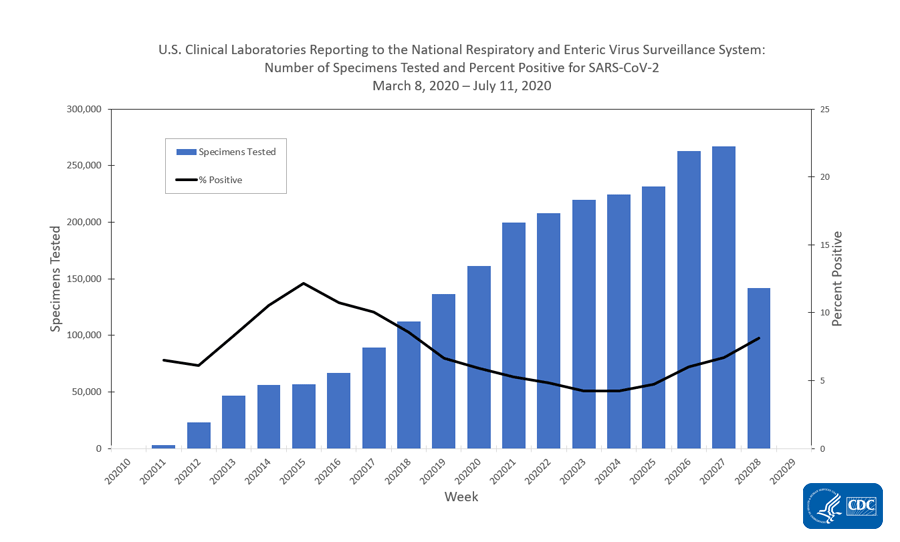 U S Clinical Laboratories Reporting Sars Cov 2 Test Results To Cdc Cdc
U S Clinical Laboratories Reporting Sars Cov 2 Test Results To Cdc Cdc
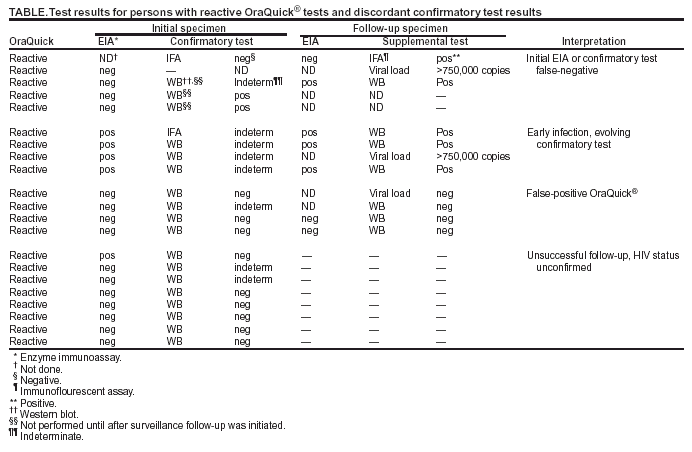 Notice To Readers Protocols For Confirmation Of Reactive Rapid Hiv Tests
Notice To Readers Protocols For Confirmation Of Reactive Rapid Hiv Tests
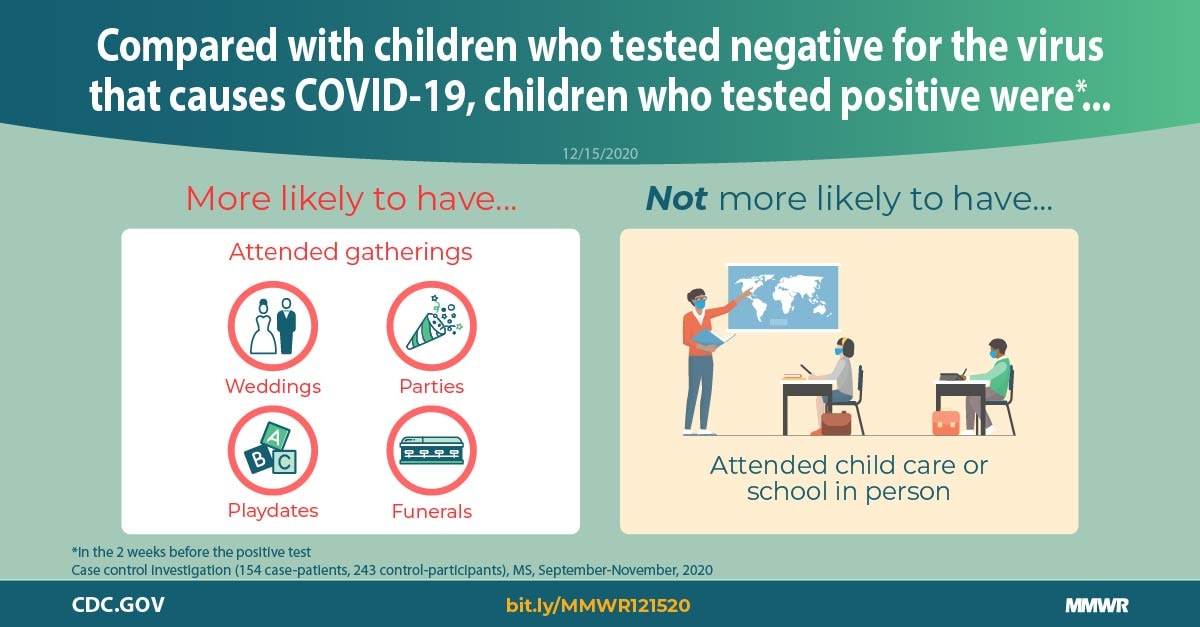 Factors Associated With Positive Sars Cov 2 Test Results In Outpatient Health Facilities And Emergency Departments Among Children And Adolescents Aged 18 Years Mississippi September November 2020 Mmwr
Factors Associated With Positive Sars Cov 2 Test Results In Outpatient Health Facilities And Emergency Departments Among Children And Adolescents Aged 18 Years Mississippi September November 2020 Mmwr
Https Www Cdc Gov Coronavirus 2019 Ncov Downloads Young Mitigation Recommendations And Resources Toolkit 02 Hs Pdf
 3 Key Steps To Take While Waiting For Your Covid 19 Test Result
3 Key Steps To Take While Waiting For Your Covid 19 Test Result
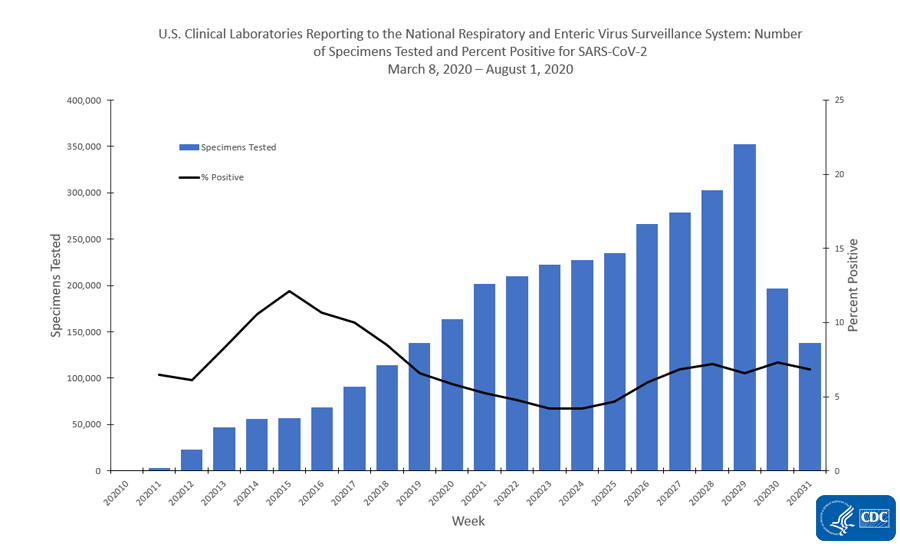 U S Clinical Laboratories Reporting Sars Cov 2 Test Results To Cdc Cdc
U S Clinical Laboratories Reporting Sars Cov 2 Test Results To Cdc Cdc
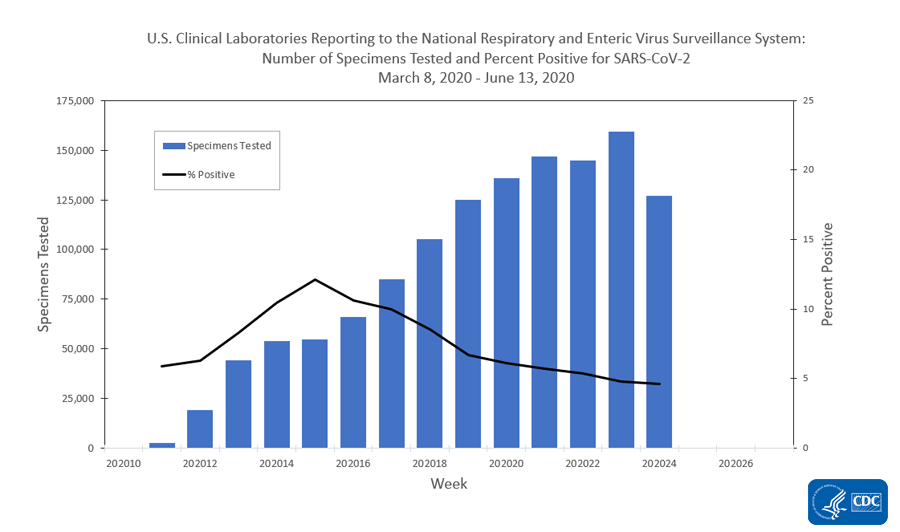 U S Clinical Laboratories Reporting Sars Cov 2 Test Results To Cdc Cdc
U S Clinical Laboratories Reporting Sars Cov 2 Test Results To Cdc Cdc
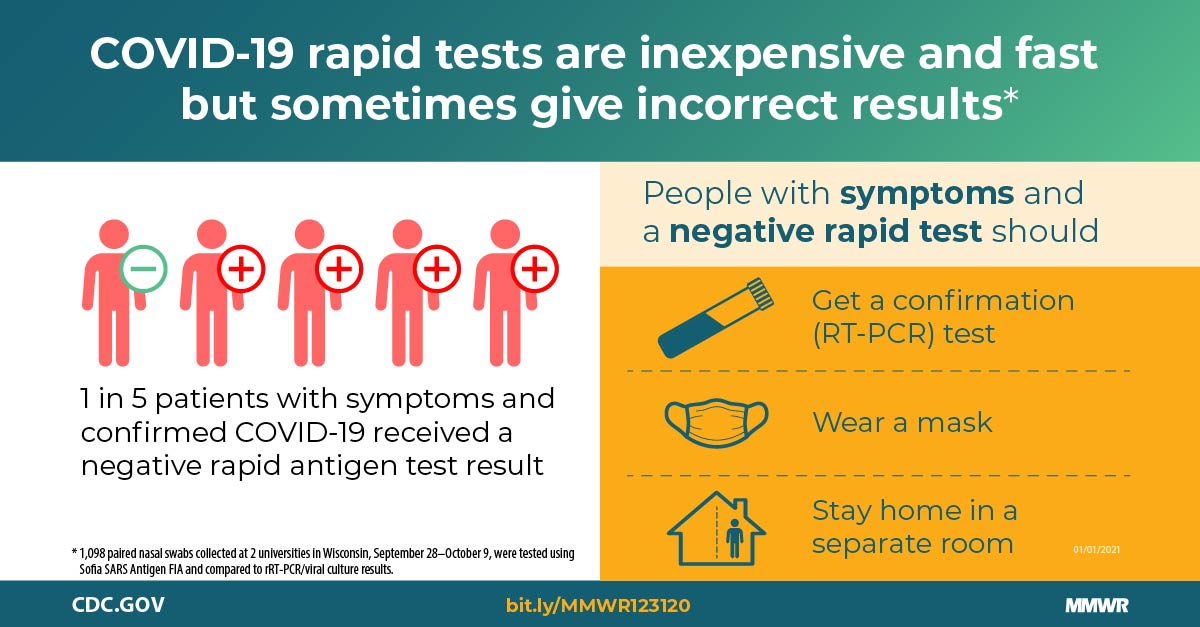 Performance Of An Antigen Based Test For Asymptomatic And Symptomatic Sars Cov 2 Testing At Two University Campuses Wisconsin September October 2020 Mmwr
Performance Of An Antigen Based Test For Asymptomatic And Symptomatic Sars Cov 2 Testing At Two University Campuses Wisconsin September October 2020 Mmwr
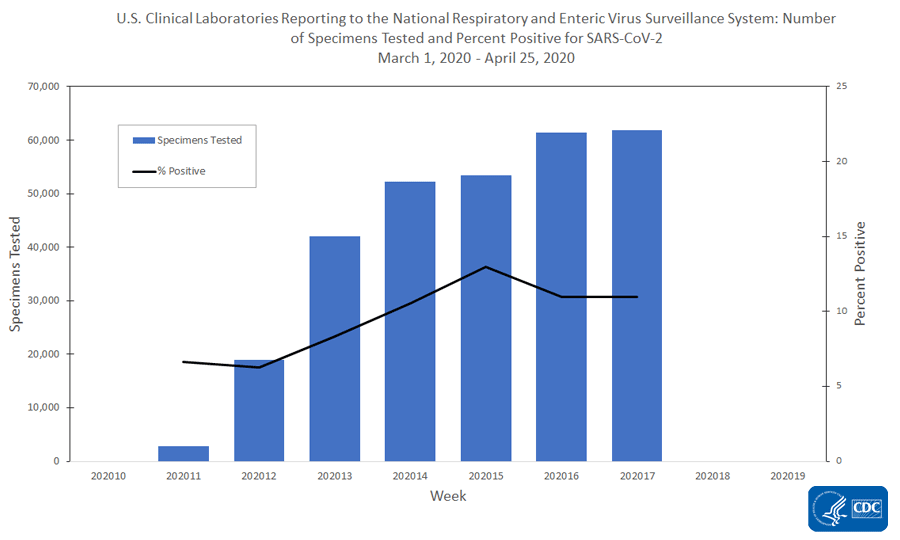 U S Clinical Laboratories Reporting Sars Cov 2 Test Results To Cdc Cdc
U S Clinical Laboratories Reporting Sars Cov 2 Test Results To Cdc Cdc

No comments:
Post a Comment
Note: Only a member of this blog may post a comment.