When signs of diabetic retinopathy are present the system recommends a follow-up with an ophthalmologist. All of this happens without input from a clinician or the services of a medical laboratory.
 Idx Dr Uses Artificial Intelligence In Medical Diagnosis For Diabetes Patients
Idx Dr Uses Artificial Intelligence In Medical Diagnosis For Diabetes Patients
To address the issue of patient compliance with diabetic retinopathy screening IDx-DR was developed as an automated screening device designed to analyze fundus images for the presence of lesions and other disease features associated with diabetic retinopathy.

Idx diabetic retinopathy. The FDA has permitted the marketing of IDx-DR the first artificial intelligence medical device to detect more than a mild level of diabetic retinopathy in adults with diabetes according to a. The IDx-DR system above delivers a binary result. Agreement between specialists was calculated.
IDx-DR a device that was previously covered on DocWire News is capable of diagnosing diabetic retinopathy without human intervention. Automated Screening for Diabetic RetinopathyDetection of Cognitive Impairment and Dementia With Artificial Intelligence. IDx-DR for Diabetic Retinopathy Screening.
IDx-DR should not be used in patients with diabetes who are pregnant. Retinal images of persons treated by the Hoorn Diabetes Care System DCS were graded by the IDx-DR device and independently by three retinal specialists using the International Clinical Diabetic Retinopathy severity scale ICDR and EURODIAB criteria. The system is the first FDA-approved autonomous artificial intelligence AI using its software to analyze images from a.
IDx-DR for Diabetic Retinopathy Screening Accuracy. IDx-DR is an AI diagnostic system that autonomously diagnoses patients for diabetic retinopathy and macular edema With IDx-DR you get. It provides instantaneous point-of-care diagnostic assessment for diabetic retinopathy and macular edema using a process.
The startup was originally called IDx since its product is called IDx-DRthe DR stands for diabetic retinopathy. IDx-DR is the first and only autonomous Artificial Intelligence system for the detection and automated early diagnosis of Diabetic Retinopathy authorized by the FDA and CE marked. Digital Diagnostics formerly IDx flagship product IDx-DR is an autonomous AI system that is FDA De Novo authorized.
Essentially the tool takes images of the back of the eye analyzes them and provides a reliable diagnosis within minutes. Results of the IDx-DR device and experts were compared using sensitivity specificity. IDx-DR is intended for use by health care providers to automatically detect more than mild diabetic retinopathy mtmDR in adults diagnosed with diabetes who have not been previously diagnosed with diabetic retinopathy.
Diabetic retinopathy with the IDx-DR device in the Hoorn Diabetes Care System Amber A van der Heijden12 Michael D Abramoff345 Frank Verbraak6 Manon V van Hecke7 Albert Liem8 and Giel Nijpels12 1Department of General Practice and Elderly Care Medicine VU University Medical Centre Amsterdam the Netherlands. The IDx-DR system was studied in 819 adults with diabetes and no previous diagnosis of diabetic retinopathy. If the system detects a moderate or severe case it automatically refers the patient to a specialist.
IDx-DR for Diabetic Retinopathy Screening. IDx-DR is indicated for use by health care providers to automatically detect more than mild diabetic retinopathy mtmDR in adults diagnosed with diabetes. Diabetic retinopathy can progress very rapidly during pregnancy and IDx-DR is not intended to evaluate rapidly progressive.
The camera used in the IDx-DR system is easy to learn and staff members can be taught to. If it detects no signs of the condition the system recommends a follow-up screening in one year. 1Lewis Katz School of Medicine at Temple University Philadelphia PA USA.
Diagnostic results at the point of care. IDx-DR is an AI diagnostic system that autonomously analyzes images of the retina for signs of diabetic retinopathy.
 Topcon Healthcare Topcon S Harmony Imaging Platform Users In Europe Now Have Access To Idx Dr
Topcon Healthcare Topcon S Harmony Imaging Platform Users In Europe Now Have Access To Idx Dr
 Pivotal Trial Results Behind The Fda S First Ever Clearance Of An Autonomous Ai Diagnostic System Published In Nature Digital Medicine
Pivotal Trial Results Behind The Fda S First Ever Clearance Of An Autonomous Ai Diagnostic System Published In Nature Digital Medicine
 Fda Approves First Ai Device To Detect Diabetic Retinopathy Medical Technology Issue 7 May 2018
Fda Approves First Ai Device To Detect Diabetic Retinopathy Medical Technology Issue 7 May 2018
 Idx And Topcon Join Forces To Scale Ai Based Diagnostic Platform In The U S Market Https Hitconsultant Net Healthcare Technology Diabetes Care Health Care
Idx And Topcon Join Forces To Scale Ai Based Diagnostic Platform In The U S Market Https Hitconsultant Net Healthcare Technology Diabetes Care Health Care
 Fda Approves Marketing For First Ai Device For Diabetic Retinopathy Detection Eyewire News
Fda Approves Marketing For First Ai Device For Diabetic Retinopathy Detection Eyewire News
Fda Clears Ai Device For Diagnosis Of Diabetic Retinopathy Is This Favorable For Use Of Ai In Digital Pathology Dark Daily
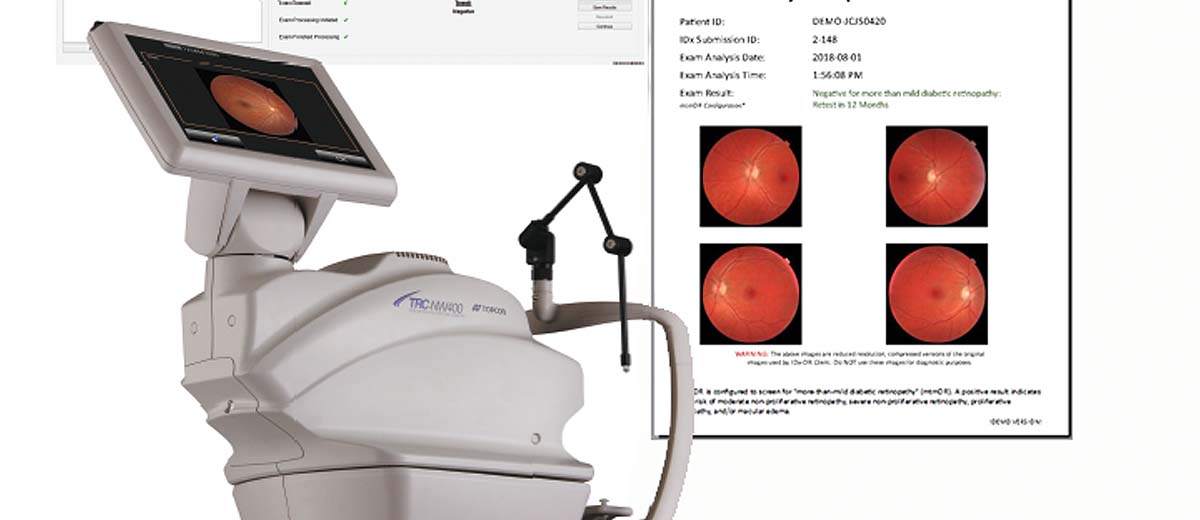 Fda Permits Marketing Of Idx Dr For Automated Detection Of Diabetic Retinopathy In Primary Care Digital Diagnostics
Fda Permits Marketing Of Idx Dr For Automated Detection Of Diabetic Retinopathy In Primary Care Digital Diagnostics
 Ai Diagnostic Tool Plows Through Fda Clearance But Some Experts Not Convinced Healthcare It News
Ai Diagnostic Tool Plows Through Fda Clearance But Some Experts Not Convinced Healthcare It News
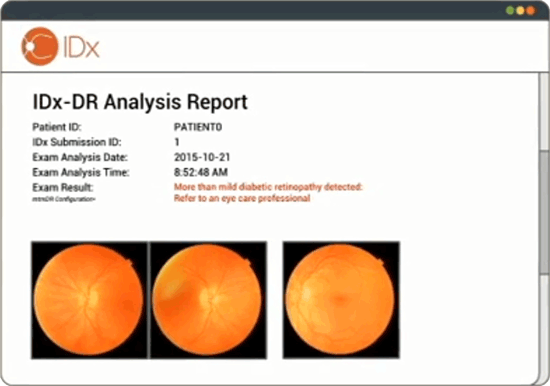 In The Us For The First Time Ai Was Allowed To Diagnose People Without Doctors Sudo Null It News
In The Us For The First Time Ai Was Allowed To Diagnose People Without Doctors Sudo Null It News
 Idx Dr Idx De Ceunynck Ophthalmology
Idx Dr Idx De Ceunynck Ophthalmology
 Ai Device That Detects Diabetes Related Eye Disease Gains Fda Approval
Ai Device That Detects Diabetes Related Eye Disease Gains Fda Approval
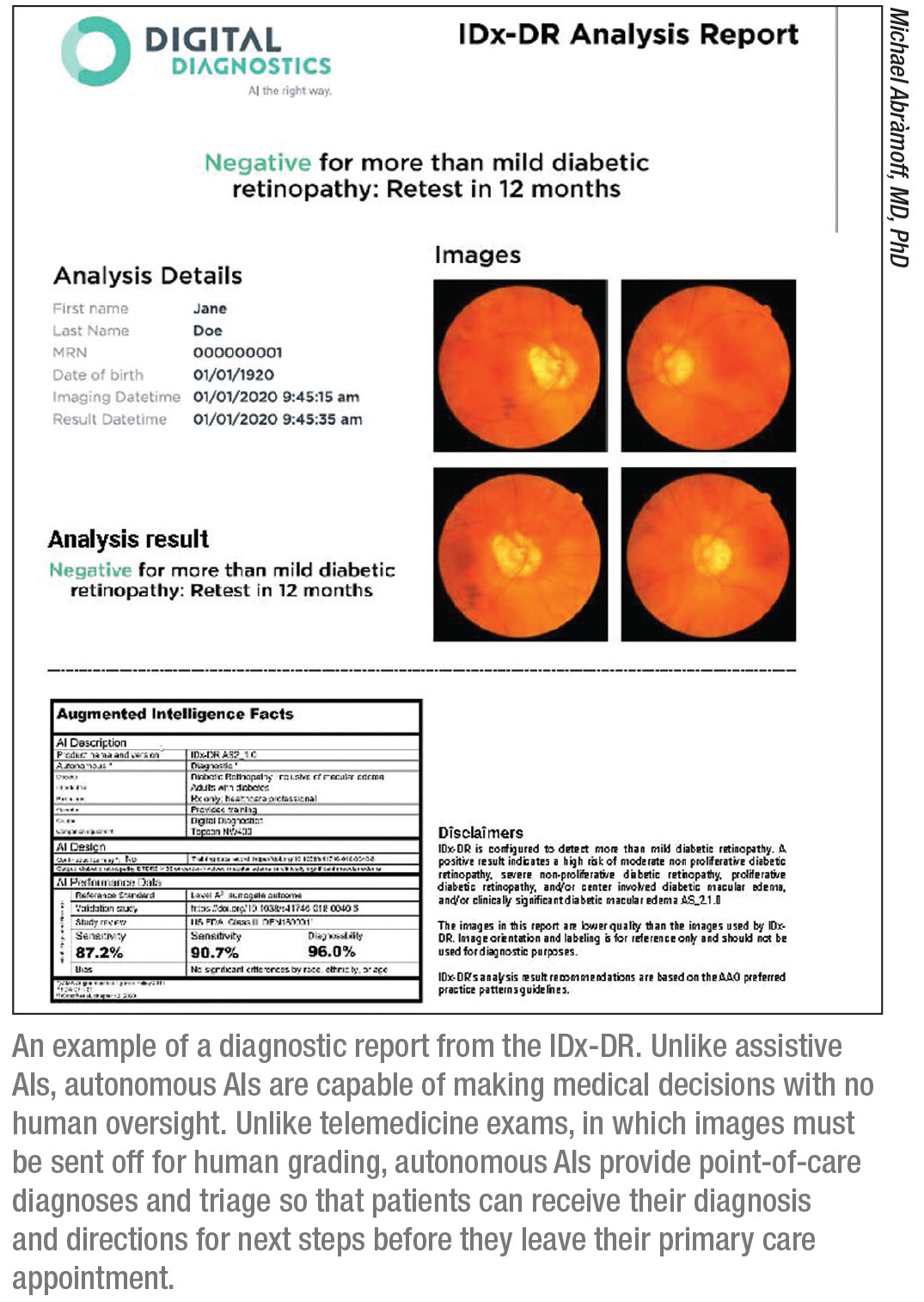 Ophthalmologists In The Machine The Ai Era
Ophthalmologists In The Machine The Ai Era
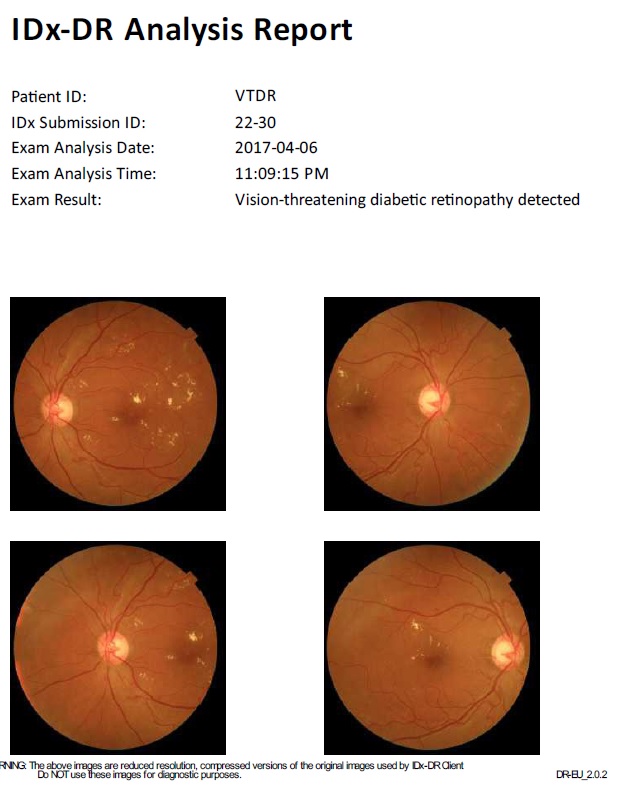

No comments:
Post a Comment
Note: Only a member of this blog may post a comment.