Staff trust and acceptance of ReNus reprocessed devices are assured as devices look brand new and are guaranteed to. Were a global leader in the manufacture supply and continuing innovation of biological and chemical indicators for sterilisation monitoring.
 The Medical Device Reprocessing Industry Third Time S The Charm
The Medical Device Reprocessing Industry Third Time S The Charm
About 315bn of single-use medical devices are sold annually in US hospitals and surgery centres of which around 150m are recycled according to Ascent Healthcare Solutions a leading reprocessing company that has recently been acquired by Stryker an orthopedic OEM.
Medical device reprocessing companies. Were leading New Zealands healthcare system to a more sustainable future. In China reprocessing and reuse of single-use medical devices SUDs are banned. A pilot survey Duojin Wang1 and Jing Wu2 Abstract Background.
RMD reusable medical device Medical device that is designated or intended by the manufacturer for use during consecutive medical. Reprocessing and reuse of single-use medical devices in China. Reprocessing of Reusable Medical Devices.
Reusable medical devices are devices that health care providers can reprocess and reuse on. The study aims to clarify the perceptions and concerns of various. Reprocessing of medical devices.
Reprocessor Person or legal entity that reprocesses used SUDs for the purpose of being used again including hospitals. CAMDR collaborates with other national and international organizations to provide its members with professional development and to elevate the quality and standards. However the actual situation has not been reported so far.
Sterilisation monitoring provides the confidence of knowing your surgical devices have been properly sterilised. ReNu Medicals reprocessing technology provides 2-3 times additional cost savings over a sterilizing reprocessors toxic EtO process. US HYGIA Health Services Inc.
In its second move to confound device reprocessing the medical device OEMs launched campaigns aimed at forcing the FDA to regulate it. It includes its cleaning disinfection sterilisation and related procedures as well as testing and restoring the technical and functional safety of the used device. The first step in beginning to reprocess medical devices is creating transparency around the supply chain situation and making sure all of the stakeholders from leadership to clinical staff are on the same page and aware of the strategy and its benefitsThe second step is to research and understand the market.
Having played a key role in the establishment of the reprocessing industry AMDR continues to push the medical technology industry and lead the way for reprocessing to play a defining role in the evolution and utilization of new device technologies. Reprocessing refers to a process carried out on a used device in order to allow its safe reuse. At 3M we understand the challenges you face in instrument reprocessing.
Reprocessing approved single-use medical devices has been cited as an effective sustainability project by Health Research Educational Trust 4 and Infection Control Today. According to the Regulation EU 2017745 on medical. The major players in the global medical device reprocessing market are Stryker Corporation US Johnson Johnson US Vanguard AG Germany Medline ReNewal US Medtronic plc Ireland SteriPro Canada IncCanada Pioneer Medical Devices AG Germany Vascular Solutions Inc.
As the trade association for third-party reprocessors AMDR focuses on providing value to the healthcare sector through reprocessing. 5 As the Original Equipment Manufacturer for many of the devices offered by reprocessors Johnson Johnson Medical Devices Companies is uniquely positioned to be your reprocessing partner of choice. Strykers Sustainability Solutions SSS is the leading provider of reprocessing and re-manufacturing services for single-use medical devices.
For questions regarding devices regulated by the Center for Devices and Radiological Health contact the Infection Control Devices Branch INCB at 301 796-5580. The OEMs attacked hospital practices claiming that their products were being recycled beyond acceptable limits and lacked appropriate quality control. Canadian Association of Medical Device Reprocessing CAMDR is a national voice and leader in Medical Device Reprocessing MDRpractices.
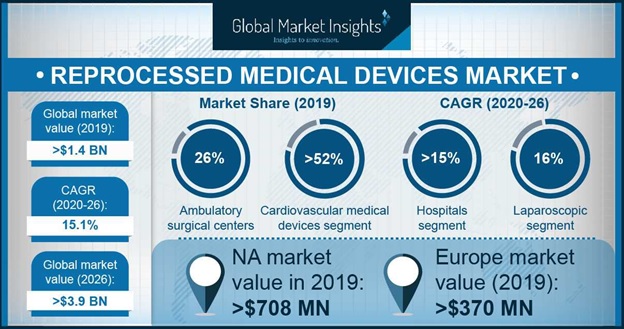
The reprocessed medical devices market size valued at USD 14 billion in 2019 and is expected to witness over 151 CAGR between 2020 and 2026 propelled by increasing number of cardiac surgeries coupled with extensive utilization of reprocessed products in cardiovascular surgeries and diagnostics. Substantial savings generated through your reprocessing programs can be reinvested into advanced patient care initiatives meaning you can treat more patients with better equipment. Thus ongoing efforts by key companies in the medical devices market to manufacture reprocessed medical devices use of new technologies for reprocessing methods will further boost uptake of reprocessed medical devices in near future.
In 2018 Sustainable Technologies joined the Association of Medical Device Reprocessors AMDR. The manufacturer that produces the medical device and places that medical device on the market. Also the company diverted 59964 pounds of waste from landfills.
Get in touch to set up a program at your hospital.
 Global Single Use Medical Device Reprocessing Market Trends
Global Single Use Medical Device Reprocessing Market Trends
 Medical Device Reprocessing Market Growing At A Cagr Of 16 3 Marketsandmarkets
Medical Device Reprocessing Market Growing At A Cagr Of 16 3 Marketsandmarkets
 Sustainability Through Reprocessing J J Medical Devices
Sustainability Through Reprocessing J J Medical Devices
 Circular Business Models In The Medical Device Industry Paths Towards Sustainable Healthcare Sciencedirect
Circular Business Models In The Medical Device Industry Paths Towards Sustainable Healthcare Sciencedirect
 Breaking Point Should We Be Reusing More Medical Devices Medical Technology Issue 10 November 2018
Breaking Point Should We Be Reusing More Medical Devices Medical Technology Issue 10 November 2018

Https Ati Ec Europa Eu Sites Default Files 2020 05 Analytical Report Nr4 Refurbishment Final Pdf
 Overview Of Medical Device Reprocessing Download Scientific Diagram
Overview Of Medical Device Reprocessing Download Scientific Diagram
 Reprocessed Medical Devices Market Growth Statistics 2020 2026
Reprocessed Medical Devices Market Growth Statistics 2020 2026
 How Reprocessing Enables Medical Device Innovation Medical Design And Outsourcing
How Reprocessing Enables Medical Device Innovation Medical Design And Outsourcing
 How Reprocessing Medical Devices Can Save Millions While Diverting Waste
How Reprocessing Medical Devices Can Save Millions While Diverting Waste
Reaping The Benefits Reprocessing Medical Devices From The Advisory Board Company Amdr
 Global Single Use Medical Device Reprocessing Market Size Share Forecast To 2025 By Omrglobal7 Issuu
Global Single Use Medical Device Reprocessing Market Size Share Forecast To 2025 By Omrglobal7 Issuu
No comments:
Post a Comment
Note: Only a member of this blog may post a comment.