Novartis won the industrys first FDA approval for a CAR-T therapy back in August 2017 when the agency endorsed Kymriah to treat a rare form of acute lymphoblastic leukemia. Novartis CAR-T therapy leads to durable response in lymphoma study.
 Novartis Exec Says Kymriah S Ready To Square Off With Bristol Myers New Car T Rival Breyanzi Fiercepharma
Novartis Exec Says Kymriah S Ready To Square Off With Bristol Myers New Car T Rival Breyanzi Fiercepharma
In der CAR-T-Zell-Therapie werden zunächst T-Zellen aus dem Blut des Patienten gewonnen die dann im Labor gentechnisch so verändert werden dass sie chimäre Antigenrezeptoren CAR auf ihrer Oberfläche bilden die gegen krebsspezifische Oberflächenproteine gerichtet sind.
Novartis car t. All compounds are either investigational or being studied for a new uses. Since then patients with certain types of life-threatening blood cancer and extremely limited survival prospects may for the first time have an opportunity to prolong their life thanks to the CAR-T cell therapy developed by Novartis. Milone MC Fish JD Carpenito C et al.
ORLANDO Novartis has shipped CAR-T cell therapies to about 1800 people with blood cancer a noteworthy accomplishment for a personalized product made from patients own immune cells. By Eric Sagonowsky Mar 8 2021 1043am. Patientinnen und Patienten mit bestimmten lebensbedrohlichen Blutkrebserkrankungen und einer sehr eingeschränkten Überlebensperspektive können seither erstmals durch die CAR-T-Zell-Therapie von Novartis eine Chance auf.
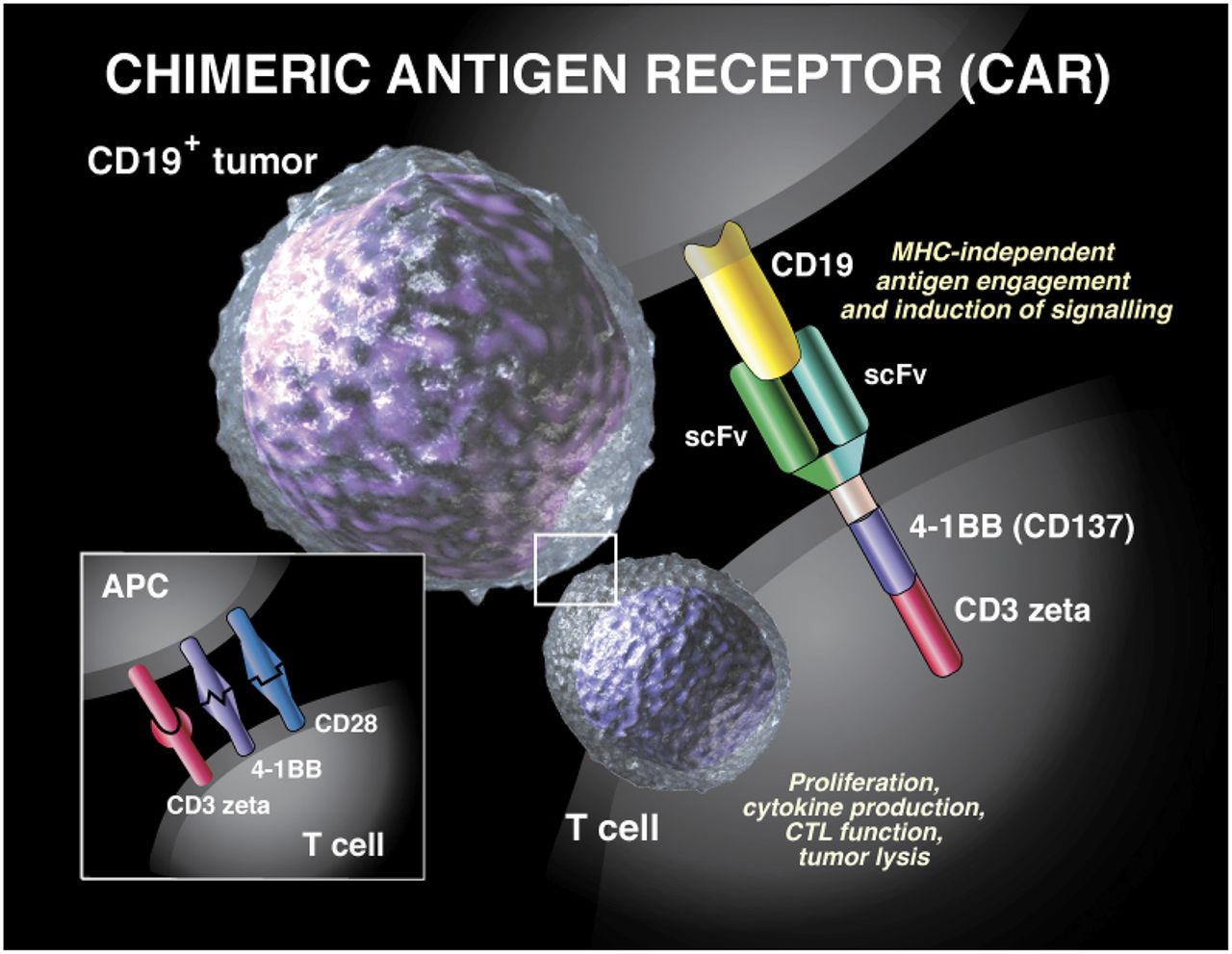
CAR-T Manufacturing at Novartis. Novartis just-approved chimeric antigen receptor CAR T-cell therapy tisagenlecleucel is going to be introduced on the market at a price of 475000 for a single infusion an amount that is. CAR-T therapy has shown impressive results treating the cancer of patients for which all other treatments fail.
Gilead which sells Yescarta for diffuse large B-cell lymphoma recently won US. There is no guarantee that they will become commercially available for the uses. Novartis Pharmaceuticals 54 Waterloo Road MACQUARIE PARK NSW 2113 Australia Telephone 61 2 9805 3555 Kymriah tisagenlecleucel CAR-T therapy from Novartis receives TGA approval for treating two aggressive blood cancers.
But some of those people have received treatments that while safe and effective dont meet commercial product specifications set out by the Food and Drug. Check out the latest CAR-T therapy research from Novartis. 3 Min Read Reuters - Patients with an advanced form of.
Manufacturing CAR-T Cell Therapies. One would expect this would translate into huge revenues for CAR-T makers but the first CAR-T Novartis Kymriah is not starting off on the right foot. Connect with Medical Information.
AACR Virtual Congress 2021. Yescarta axicabtagene ciloleucel or axi-cel for short has become the second CAR-T therapy to be approved in the USThe FDA has authorized its use to treat aggressive B-cell non-Hodgkin lymphoma in patients for whom at least two other types of treatments. Find disease education MOA resources and clinical trials on CAR-T therapy at AACR 2021.
One in multiple myeloma using two different CAR T-cell therapies. Approval for Tecartus a slightly different version of Yescarta that is now cleared for treating patients. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased anlileukemlc efficacy in vivo.
Die Immunzellen werden somit künstlich auf den Krebs abgerichtet. Currently approved CAR-T cell therapy is an individualized treatment designed specifically for each patient. Global CAR-T Therapy Pipeline Market Analysis Report 2021-2030 Featuring Novartis Kite Pharma Pfizer Juno Therapeutics Celgene CARsgen Therapeutics Sorrento Therapeutics and Legend Biotech.
Efficacy and safety have not been established. But Novartis as well as its CAR-T competitor Gilead have continued to study their therapies in new cancer types as well as in earlier lines of treatment. Vor gut einem Jahr am 27.
One trial that combines CAR T-cell therapy with. In the first quarter of 2018 the treatment brought in 12M 10M roughly. Just over a year ago on 27 August 2018 the European Commission gave their approval for the CAR-T cells developed by Novartis to be used in Germany.
August 2018 hat die Europäische Kommission die CAR-T-Zellen von Novartis in Deutschland zugelassen. Gileads CAR-T cell therapy Yescarta has been approved by the FDA making it the first competitor for Novartis CAR-T Kymriah. CAR-T cell therapy is a cutting-edge immunotherapy that uses specifically altered cells from your immune system to fight cancer in your blood.
This site is for health care professionals only. The new alliance between Novartis and Penn will focus on four clinical trials. Kymriah is an immunocellular therapy that is a one-time treatment manufactured.
Gileads CAR-T med Yescarta gets a leg up on Novartis rival with FDA nod in follicular lymphoma.
 Oxford Biomedica Signs 100m Car T Deal With Novartis
Oxford Biomedica Signs 100m Car T Deal With Novartis
 Chimeric Antigen Receptor T Cell Car T Therapy Novartis
Chimeric Antigen Receptor T Cell Car T Therapy Novartis
 Novartis Gains Access To Celyad S Allogenic Car T Cell Patents
Novartis Gains Access To Celyad S Allogenic Car T Cell Patents
 Car T Novartis Prices Ctl019 At Us 475 000 European Biotechnology
Car T Novartis Prices Ctl019 At Us 475 000 European Biotechnology
 Manufacturing Challenge Leads To Death In Car T Trial
Manufacturing Challenge Leads To Death In Car T Trial
 Novartis Matches Gilead On Price In New Car T Use
Novartis Matches Gilead On Price In New Car T Use
 Car T Therapies Are Manufactured For Each Individual Patient Novartis
Car T Therapies Are Manufactured For Each Individual Patient Novartis
 Xconomy Novartis Car T Results In One Month Or No Charge Why One Month
Xconomy Novartis Car T Results In One Month Or No Charge Why One Month
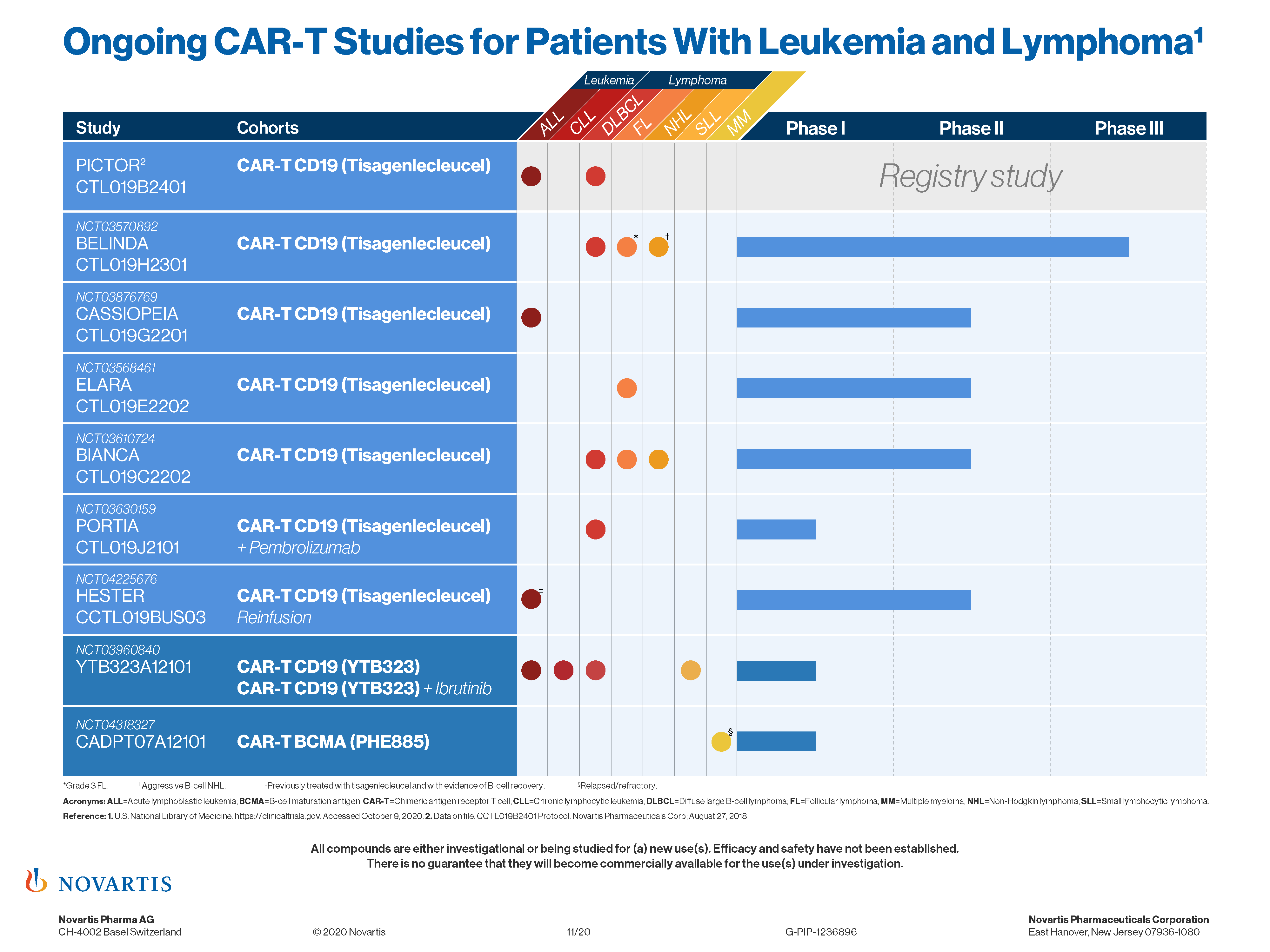
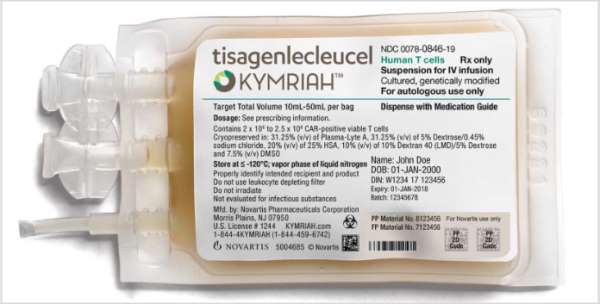
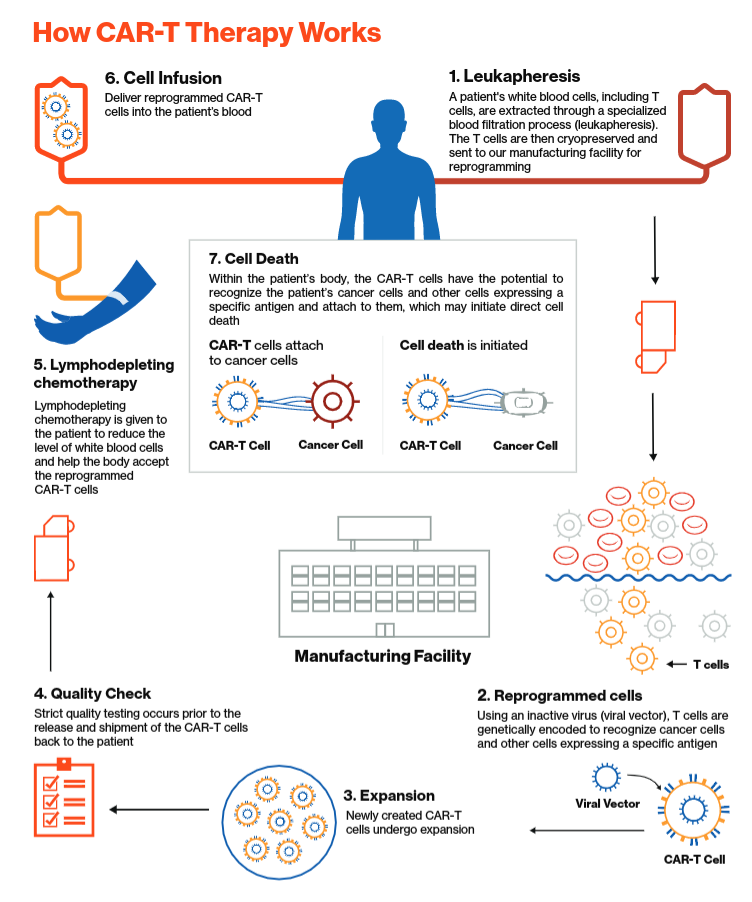
 Car T Mechanism Of Action Kymriah Tisagenlecleucel
Car T Mechanism Of Action Kymriah Tisagenlecleucel
 Optimizing Car T Cell Manufacturing Processes During Pivotal Clinical Trials Molecular Therapy Methods Clinical Development
Optimizing Car T Cell Manufacturing Processes During Pivotal Clinical Trials Molecular Therapy Methods Clinical Development
Fda Approves Novartis Car T Cancer Treatment First Gene Therapy
 Car Ts Novartis Wins Uk European Biotechnology
Car Ts Novartis Wins Uk European Biotechnology
 The Fda Approves A Second Car T Therapy Cheaper Than Novartis
The Fda Approves A Second Car T Therapy Cheaper Than Novartis
 The Novartis Commitment To Reimagining Car T Cell Therapy
The Novartis Commitment To Reimagining Car T Cell Therapy
No comments:
Post a Comment
Note: Only a member of this blog may post a comment.