Food and Drug Administration FDA has officially launched the Digital Health Center of Excellence within the Center for Devices and Radiological Health CDRH to expand the agencys activities in furtherance of its overarching dedication to the advancement of digital health technology including mobile medical devices software as a Medical Device SaMD artificial. The FDA is calling on the digital health industry to innovate and proposing a new Center of Excellence for Digital Health the timing of which is critical as the industry is receiving more funding and opportunities than ever before says industry exec.
 Digital Health Center Of Excellence Fda
Digital Health Center Of Excellence Fda
The program is part of the FDAs Center.

Fda digital health center of excellence. Last month the US Food and Drug Administration FDA launched the Digital Health Center of Excellence DHCoE bolstering the shift toward digital that the healthcare industry has already been embracing. FDA launches digital health center of excellence Posted 23 September 2020 By Kari Oakes A new digital health Center of Excellence within the US Food and Drug Administration FDA aims to boost digital health innovation by fostering partnerships and connections among digital health. The FDA will discuss the purpose and functions of the DHCoE and will focus on collecting feedback from attendees.
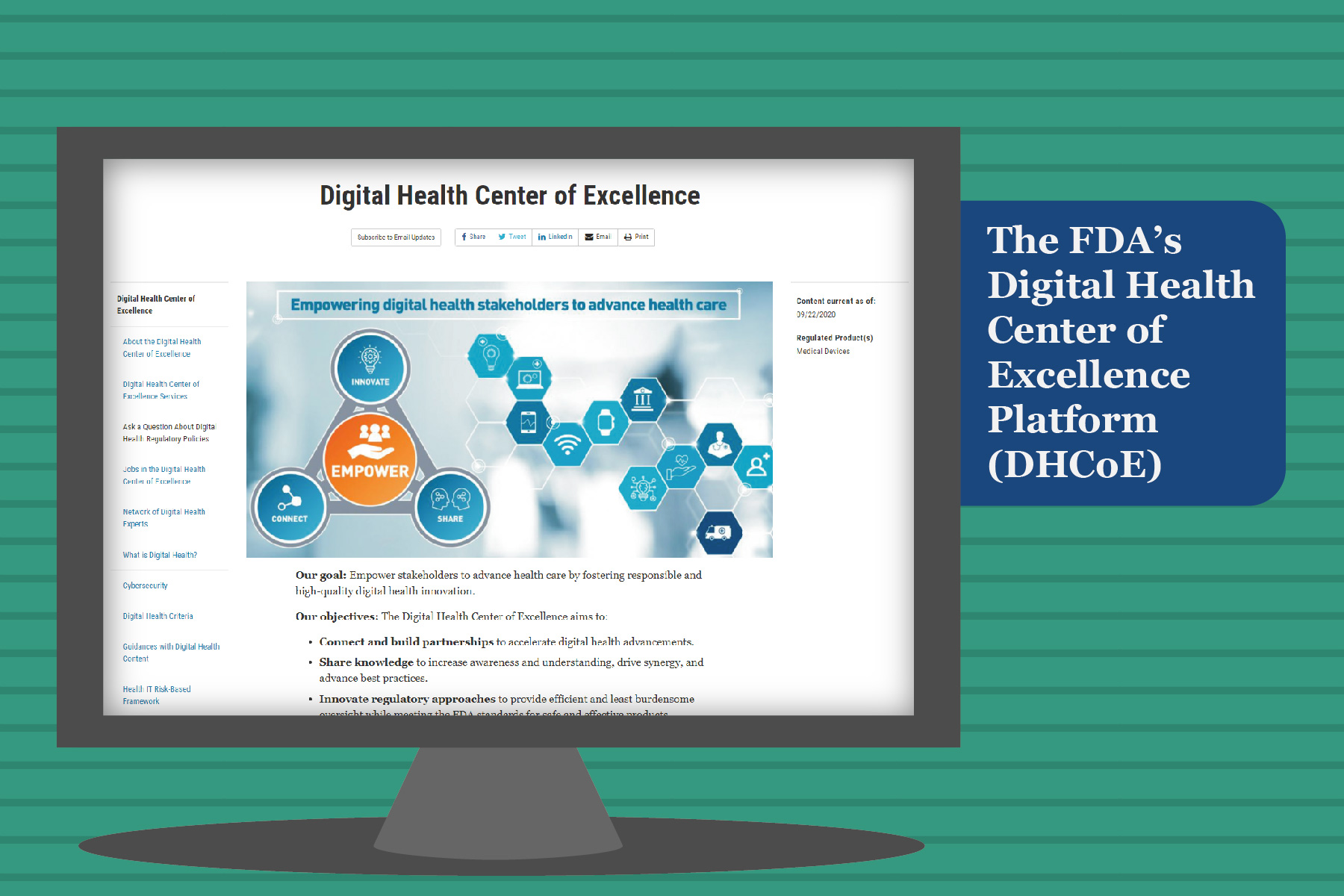
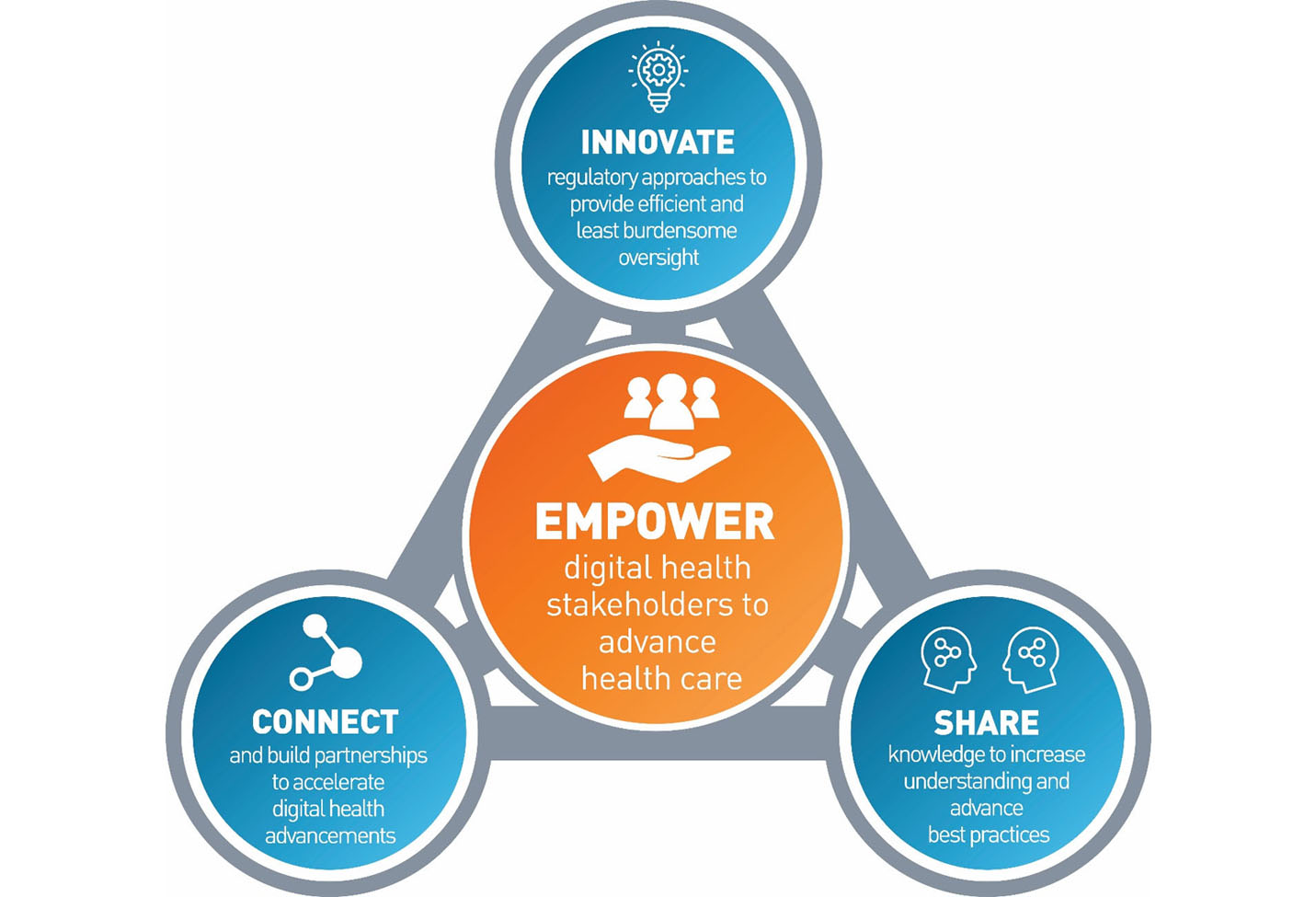
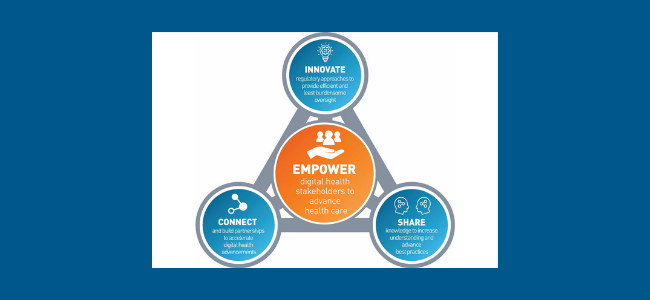
The FDA said the new Digital Health Center of Excellence will be tasked with providing technical advice advancing best practices and reimagining digital health device oversight. Investment in digital health is at an all-time high. Share knowledge to support best practices.
It is committed to strategically advancing science and evidence for digital health technologies within the framework of the FDAs regulatory and. On October 19 2020 the US. The FDA is creating a Digital Health Center of Excellence DHCEwhich will establish a dedicated regulatory framework for digital health developers and.
Establishing the Digital Health Center of Excellence is part of the FDAs work to ensure that the most cutting-edge digital health technologies are rapidly developed and reviewed in the US FDA Commissioner Stephen M. September 22 2020-- The US. The Digital Health Center of Excellence is committed to strategically advancing science and evidence for digital health technologies within the framework of the FDAs.
The Digital Health Center of Excellence is committed to strategically advancing science and evidence for digital health technologies within the framework of the FDA. The center of excellence will also work to build a network of digital health experts to help develop the FDAs priorities and launch strategic partnerships with stakeholders in the community. Housed within the Center for Devices and Radiological Health CDRH the Digital Health Center of Excellence DHCE will focus on a range of technologies.
In its September 22 announcement FDA shared with stakeholders that it had launched the Digital Health Center of Excellence DHCoE which is envisioned to provide centralized expertise and serve as a resource for digital health technologies and policy for digital health innovators the public and FDA. The Digital Health Center of Excellence provides services to various stakeholders within and outside the FDA. The center will seek to do the following.
Establishing the Digital Health Center of Excellence is part of the FDAs work to ensure that the most cutting-edge digital health technologies are rapidly developed and reviewed in the US. September 22 2020 - The US Food and Drug Administration has unveiled the Digital Health Center of Excellence aimed at supporting mHealth and telehealth tools. The FDA recently launched the Digital Health Center of Excellence DHCoE bolstering the shift toward digital that the healthcare industry has already been embracing.
Food and Drug Administration FDA will host a virtual listening session for digital health device manufacturers developers health care providers researchers and other stakeholders to learn about the Digital Health Center of Excellence DHCoE. Fast-track digital health advancements. Food and Drug Administration FDA has introduced what it is calling the Digital Health Center of Excellence intended to support high-quality digital health innovation.
Develop regulatory approaches that will offer efficient oversight while also meeting FDA standards. The Digital Health Center of Excellence will provide centralized expertise and serve as a resource for digital health technologies and policy for digital health innovators the public and FDA staff. The FDA has launched its Digital Health Center of Excellence DHCoE as the agency continues with its commitment to advancing use of technology such as.
The FDA announced today the launch of the Digital Health Center of Excellence a central resource intended to help the agency as well as external stakeholders promote digital health technologies.
 Fda Launches The Digital Health Center Of Excellence Medical Product Outsourcing
Fda Launches The Digital Health Center Of Excellence Medical Product Outsourcing
 Fda Launches Digital Health Center Of Excellence
Fda Launches Digital Health Center Of Excellence
 The Fda S Digital Health Center Of Excellence Platform Dhcoe Emma International
The Fda S Digital Health Center Of Excellence Platform Dhcoe Emma International
 Us Fda Launches The Digital Health Center Of Excellence Meditrial Helpline
Us Fda Launches The Digital Health Center Of Excellence Meditrial Helpline
 About The Digital Health Center Of Excellence Fda
About The Digital Health Center Of Excellence Fda
 Fda Launches Digital Health Center Of Excellence Medsyscon Medizintechnik Gmbh
Fda Launches Digital Health Center Of Excellence Medsyscon Medizintechnik Gmbh
 Fda Launches The Digital Health Center Of Excellence
Fda Launches The Digital Health Center Of Excellence
 Fda Launches Digital Health Center Of Excellence Healthcare Innovation
Fda Launches Digital Health Center Of Excellence Healthcare Innovation
 Fda Launches Center Of Excellence To Advance Digital Health
Fda Launches Center Of Excellence To Advance Digital Health
 Digital Health Center Of Excellence Fda
Digital Health Center Of Excellence Fda
 Fda Launches Digital Health Focused Center Of Excellence Fiercebiotech
Fda Launches Digital Health Focused Center Of Excellence Fiercebiotech
 Digital Health Center Of Excellence Services Fda
Digital Health Center Of Excellence Services Fda
 Introducing The Fda Digital Health Center Of Excellence American Gastroenterological Association
Introducing The Fda Digital Health Center Of Excellence American Gastroenterological Association
 Fda Announces Digital Health Center Of Excellence To Advance Wearables And Other Healthcare Tech Acrp
Fda Announces Digital Health Center Of Excellence To Advance Wearables And Other Healthcare Tech Acrp
No comments:
Post a Comment
Note: Only a member of this blog may post a comment.