May 05 2021 - 0847 AM The American Medical Association yesterday announced Current Procedural Terminology codes for reporting the two-dose Novavax COVID-19 vaccine and its administration on medical claims if the Food and Drug Administration approves the candidate vaccine or authorizes it for emergency use. Novavax is targeting a second-quarter FDA filing for emergency use of its COVID-19 vaccine.
 Covid Vaccine Novavax Expects Fda Clearance As Early As May
Covid Vaccine Novavax Expects Fda Clearance As Early As May
Pending approval the company has agreed to supply the US.

Novavax fda approval. IStock In late January Novavax announced its protein-based vaccine showed a 893 efficacy against coronavirus. In July the US. The goal suggests the US.
On the COVID-19 side of things Novavax has an agreement with the US. Novavax will now seek regulatory approval for its vaccine in the US the UK and in Europe in the third quarter. Delays in clinical-trial results coupled with manufacturing delays have slowed the process by which the company would seek.
Could join the UK. That at least is the opinion of Novavax NASDAQ. Government to supply 100 million doses of its vaccine.
The Food and Drug Administration FDA has approved both the vaccines from Indian biotechnology company Bharat Biotech and a subsidiary of American pharmaceutical giant Johnson Johnson for emergency use against Covid-19. For its part Novavax has a license agreement with Serum Institute to provide NVX-CoV2373. On Monday Erck said that his.
The drugmaker has assured that it will deliver 100 million doses of the vaccine to the county. The FDA could authorize Novavaxs Covid-19 vaccine for emergency use as early as May the companys CEO Stanley Erck told CNBC. Bharat Biotech International Ltds Covaxin Astrazenecas Vaxzevria AZD-1222 Serum Institute of Indias Covishield AZD-1222 and the Gamaleya National Centre of Epidemiology and Microbiologys Sputnik V.
The company hopes the FDA will allow it to use data from its. Novavax CEO Says Its Coronavirus Vaccine Could Win FDA Approval in May Eric Volkman 322021. Trial is expected to report results this quarter and an FDA.
FDA Director-General Rolando Enrique Domingo said the two-dose Covaxin from India and the JNJ-78436735 or Janssen single-dose. Novavax seeks FDA emergency approval for COVID-19 vaccine by May Company is hoping FDA will review data from a UK-based trial Novavaxs COVID-19 vaccine could receive an emergency use approval from the US Food and Drug Administration FDA in May according to the companys chief executive officer Stanley Erck. Might be only a few weeks away from getting a fourth authorized coronavirus vaccine.
On Monday Erck said. NVAX bullish thesis Novavax NVAX s share price has more than doubled since the excellent UK trial data came. Granted 16 billion to Novavax to support its COVID-19 vaccine candidate.
That at least is the opinion of Novavax NASDAQNVAX CEO Stanley Erck. Vaccine manufacturer Novavax said on Monday that it plans to apply for the Food and Drug Administrations emergency authorization in the third quarter of 2021 pushing back previous predictions it. The biotech company had previously projected potential US authorization in May.
The sooner-than-expected FDA approval can drive Novavaxs share price even higher. Novavax reports first-quarter earnings on Monday after the closing bell. With 110 million doses.
Novavax will receive royalties on all sales of the vaccine if approved. 34 rows NVX-CoV2373 FDA Approval Status. Four vaccines are approved in India.
Novavax shares on Tuesday sharply extended losses during a two-day rout after the biotech company delayed seeking approval for its COVID-19 vaccine from three regulators including the Food and. NVAX CEO Stanley Erck. Novavax launched that trial in December and aims to include 30000 volunteers across more than 100 sites.
Its very likely the FDA will. Novavaxs vaccine officially known as NVX-CoV2373 is protein-based. Novavax has promised to deliver 350 million doses to COVAX which provides shots to low- and middle-income countries beginning in the third quarter of the year and 11.
Billion doses over time.
 Novavax Covid 19 Vaccine 90 Efficacious In Phase 3 But Protection Plummets Against One Variant Fiercebiotech
Novavax Covid 19 Vaccine 90 Efficacious In Phase 3 But Protection Plummets Against One Variant Fiercebiotech
 Novavax Sees U K Vaccine Approval First In Talks With Fda Bloomberg
Novavax Sees U K Vaccine Approval First In Talks With Fda Bloomberg
 Novavax To Seek Eua For Covid 19 Vaccine As Early As April
Novavax To Seek Eua For Covid 19 Vaccine As Early As April
 Novavax Vaccine Trial Prepares For Fda Approval 11alive Com
Novavax Vaccine Trial Prepares For Fda Approval 11alive Com
 Novavax Covid 19 Vaccine Performs Well In Clinical Trials But Variants Remain A Threat
Novavax Covid 19 Vaccine Performs Well In Clinical Trials But Variants Remain A Threat
 Novavax Says Its Covid 19 Vaccine Is 90 Effective In Late Stage Trial
Novavax Says Its Covid 19 Vaccine Is 90 Effective In Late Stage Trial
 Novavax S Nvx Cov2373 Will Still Be The Second Leading Candidate Despite Delays
Novavax S Nvx Cov2373 Will Still Be The Second Leading Candidate Despite Delays
 I M In A Covid 19 Vaccine Trial But Maybe Not For Long Medpage Today
I M In A Covid 19 Vaccine Trial But Maybe Not For Long Medpage Today
 Novavax In Talks With Fda Over Quick Approval For Covid 19 Shot
Novavax In Talks With Fda Over Quick Approval For Covid 19 Shot
 Novavax Swamped With Fda S Manufacturing Questions Hopes For U S Coronavirus Shot Trial Within The Year Fiercepharma
Novavax Swamped With Fda S Manufacturing Questions Hopes For U S Coronavirus Shot Trial Within The Year Fiercepharma
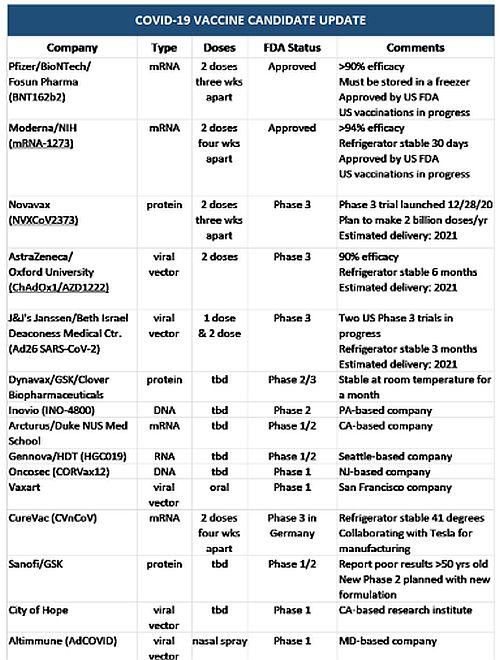 The Lead Covid 19 Vaccine Candidates Where We Are And What You Should Know Coronavirus Covid 19 United States
The Lead Covid 19 Vaccine Candidates Where We Are And What You Should Know Coronavirus Covid 19 United States
Novavax Seeks Fda Emergency Approval For Covid 19 Vaccine By May Pmlive
 Novavax Targets May Approval For Covid 19 Vaccine In The U S Fiercebiotech
Novavax Targets May Approval For Covid 19 Vaccine In The U S Fiercebiotech
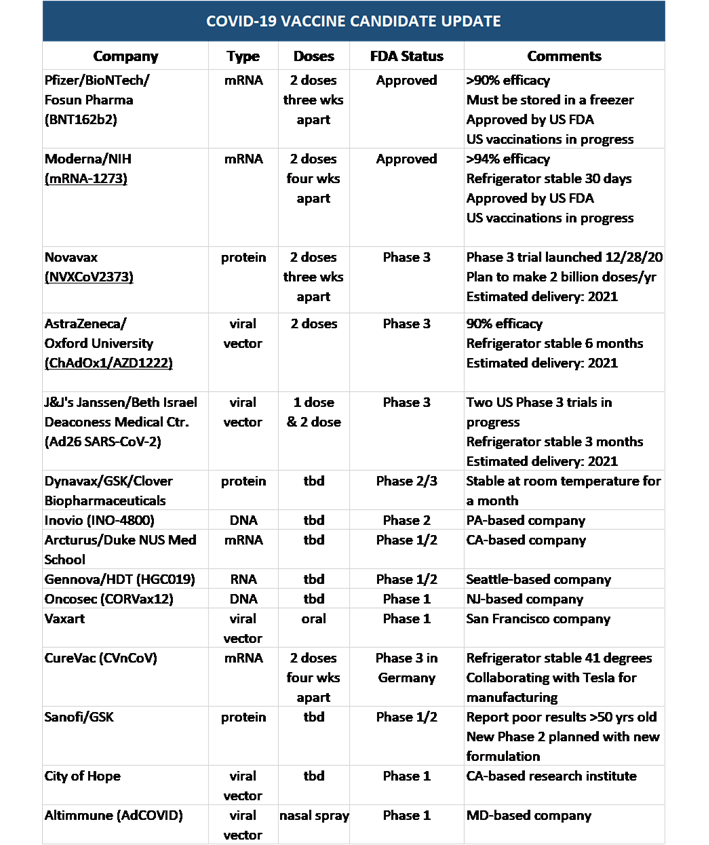 The Lead Covid 19 Vaccine Candidates Where We Are And What You Should Know Lexology
The Lead Covid 19 Vaccine Candidates Where We Are And What You Should Know Lexology
No comments:
Post a Comment
Note: Only a member of this blog may post a comment.