July 21 2020 - USP Stakeholder Engagement Plan on Beyond-Use Date BUD Provisions in General Chapters Registration opened for September 15 2020 Open Forum June 26 2020 Revision Bulletin published to clarify the term antineoplastic for the purpose of Chapter. Piervincenzi PhD chief executive officer USP.
 The Pcca Blog Recommendations For Navigating Usp 800
The Pcca Blog Recommendations For Navigating Usp 800
USP is relevant to all healthcare facilities and personnel who prepare transport or administer hazardous drugs regardless of the volume being handled or the size of the facility.
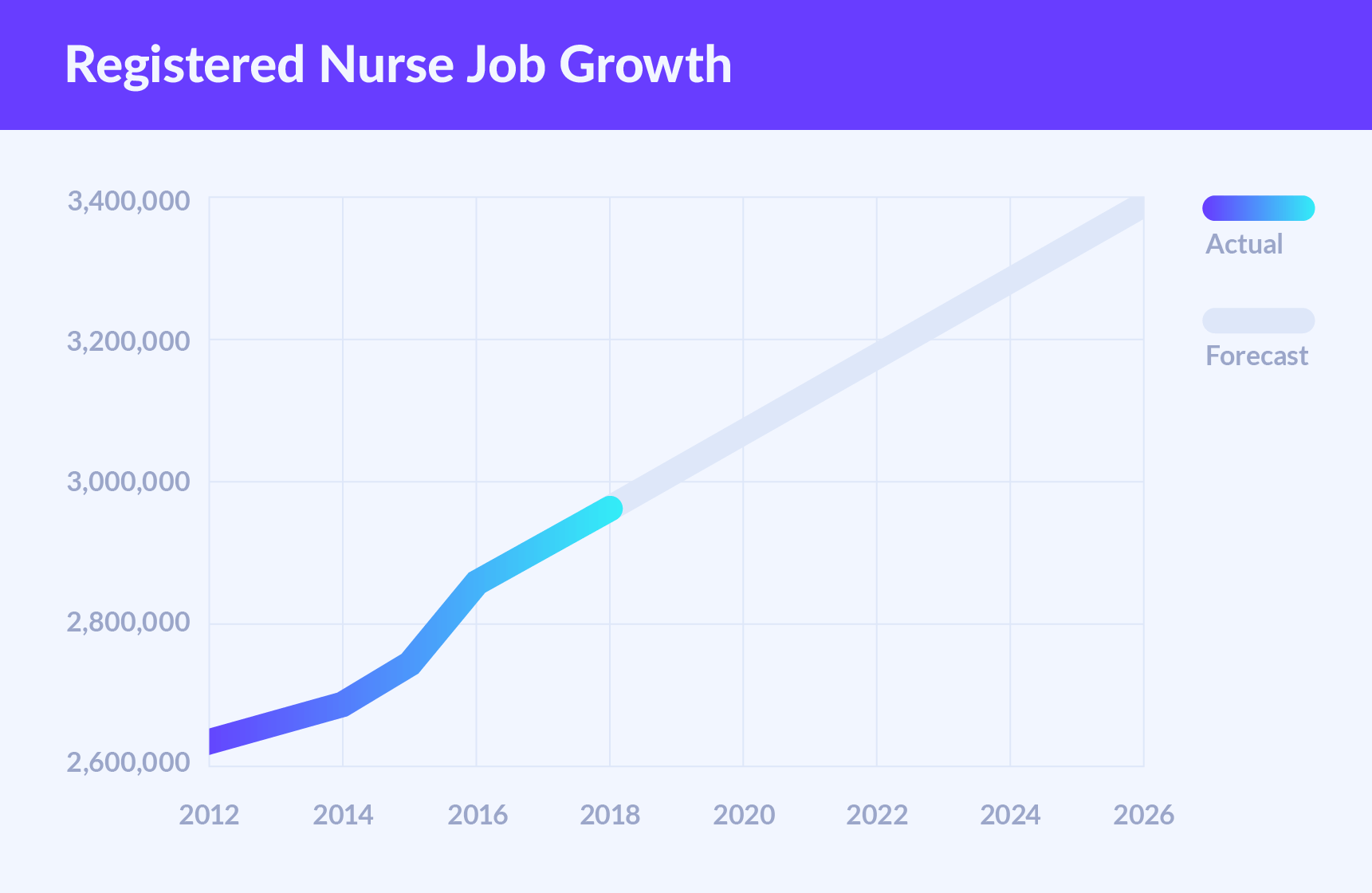
Usp 800 guidelines 2020. Many organizations are or will soon be fully compliant with the 2019 USP revisions. 795 Pharmaceutical Compounding Nonsterile Preparations Reprinted from USP42-NF37 last revised 2014 797 Pharmaceutical Compounding Sterile Preparations Reprinted from USP42-NF37 last revised 2008 800 Hazardous Drugs Handling in Healthcare Settings Informational 825 Radiopharmaceuticals Preparations Compounding. USP was scheduled to go into effect on Dec.
In response to peer reviews and public comments NIOSH proposes a reorganization of the tables in the draft 2020 List in a manner that may address at least some of the concerns expressed. Procedures for deactivating decontaminating and cleaning. Stay informed on USP Compounding Standards by signing up for USP updates.
USP 800 describes practice and quality standards for the handling of HDs involving but not limited to the receipt storage compounding dispensing administration and disposal of sterile and non-sterile products. The United States Pharmacopeia USP was created over 200 years ago dedicated to instilling trust where it matters most. Pharmacopeia USP Convention Chapter 800.
USP is intended to provide a standardized guideline because tight control of each step in the HD life cycle is necessary. In the medicines supplements and foods people rely on for their health. It is available from the.
Yet the regulatory bodies will be surveying on the current versions with a few exceptions at the state level or even by a section of the USP Chapters. USP Hazardous DrugsHandling in Healthcare Settings1 was published in the First Supplement to USP 39NF 34 on February 1 2016 with an extended official date of July 1 2018. One erratum was published on April 15 20162 Pharmacies and other entities where handling hazardous drugs HDs occur should obtain a copy of the full document.
Overall properly educating pharmacy workers and phy- siciansas well as risk management legal and drug delivery personnelis crucial in providing quality protection to patients and their health care team. USP requires covered workplaces to maintain an internal list of HDs used in their facilities and review that list at least every 12Geschätzte Lesezeit. To align with the revisions to Pharmaceutical CompoundingNonsterile Preparations and.
With the potential implementation of USP in 2020 it is imperative that human resource leaders familiarize themselves with the health care worker protection requirements associated with exposure to hazardous drugs and partner with their leadership teams to. USP Requirements for Maintaining an Inventory of Hazardous Drugs. In the future if the revised USP and contain reference to USP would be applicable and compendially required only to the extent to which USP General Chapters and apply.
TIFS EC conducted a clinically-based review of Part D drugs approved since Version 50 and updated the USP Categories and Classes to accommodate changes in. USP defines HDs according to the criteria established in the NIOSH List of Antineoplastic and Other Hazardous Drugs in Healthcare Settings 2016. In accordance with the Rules and Procedures of the 20152020 Council of Experts USP revised the official date of Hazardous DrugsHandling in Healthcare Settings to December 1 2019.
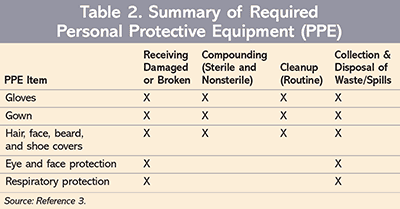
Facility and engineering controls. The official date of was previously postponed. This chapter applies to any personnel who may be exposed to HDs.
The quality standards we develop help manufacturers deliver on their promises of safe products while building confidence among healthcare practitioners patients and consumers. These standards apply to all healthcare personnel who receive prepare administer transport or otherwise. According to Polovich and Olsen 2017 The implementation of the USP Standards will represent an important step forward for nurses and other potentially exposed HCWs p.
All of the recommendations for hazardous drug handling are defined in USP which provides references to other source documents. Handling in Healthcare Settings USP 2016 on December 1 2019. USP General Chapter describes requirements including responsibilities of personnel handling hazardous drugs.
Since administration is out of scope of USP. For hazardous drugs this means only when a practitioner is compounding as that term is defined in USP and would be applicable and compendially required. Standard setting process through the 2020-2025 Call for Candidates.
USP Therapeutic Information and Formulary Support Expert Committee TIFS EC has developed the USP Medicare Model Guidelines v60 available for use by Part D plans for benefit years 20152017. During this appeals process USP will be informational and not compendial applicable. USP General Chapter Hazardous DrugsHandling in Healthcare Settings is another relevant chapter for compounding to help promote the safe handling of hazardous drugs by healthcare workers.
1 2019 but the US. USP encourages utilization of in the interest of advancing public health. USP added clarification about the application of chapter to hazardous drugs which can be found on its FAQ page.
From the time a medication is compounded until the moment the patient takes it we want to help ensure patient benefit and reduce risks said Ronald T. Pharmacopeia postponed enforcement while appeals on related provisions in USP and are resolved. While 2020 brought some progress and clarity for both the revision of the USP compounding chapters and the long-awaited NIOSH list of hazardous drugs the reality is that the official versions of these guiding documents are dated.
Because the way cancer is treated therapeutically has changed and the classes of drugs used to fight cancer have changed antineoplastic drugs.